Page 109 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 109
86 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
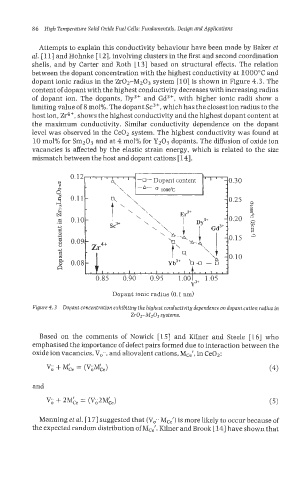
Attempts to explain this conductivity behaviour have been made by Baker et
al. [ 111 and Hohnlce [ 121, involving clusters in the first and second coordination
shells, and by Carter and Roth [13] based on structural effects. The relation
between the dopant concentration with the highest conductivity at 1000°C and
dopant ionic radius in the Zr02-M203 system [lo] is shown in Figure 4.3. The
content of dopant with the highest conductivity decreases with increasing radius
of dopant ion. The dopants, Dy3+ and Gd3+, with higher ionic radii show a
limiting value of 8 mol%. The dopant Sc3+, which has the closest ion radius to the
host ion, Zr4+, shows the highest conductivity and the highest dopant content at
the maximum conductivity. Similar conductivity dependence on the dopant
level was observed in the Ce02 system. The highest conductivity was found at
10 mol% for Sm203 and at 4 mol% for Yz03 dopants. The diffusion of oxide ion
vacancies is affected by the elastic strain energy, which is related to the size
mismatch between the host and dopant cations [14].
Dopant ionic radius (0.1 nm)
Figure 4.3 Dopant concentration exhibiting the highest conductivity dependence on dopant cation radius in
Zr02-iU203 systems.
Based on the comments of Nowiclc [15] and Kilner and Steele [16] who
emphasised the importance of defect pairs formed due to interaction between the
oxide ion vacancies, V;., and aliovalent cations, Mc,', in CeOz:
and
V; + 2Mhe = (V;2MLe)
Mar,ning et al. [17] suggested that (V;. Mce/) is more likely to occur because of
the expected random distribution ofMc,'. ICilner and Brook [14] have shown that