Page 114 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 114
Electrolytes 91
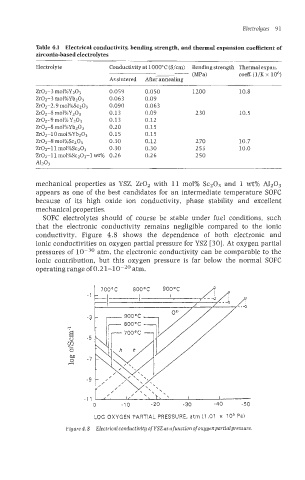
Table 4.1 Electrical conductivity, bending strength, and thermal expansion coefficient of
zirconia-based electrolytes
Electrolyte Conductivity at 1000°C (S/cm) Bending strength Thermal expan.
(MW coeff. (1/K x lo6)
As sintered After annealing
Zr02-3 mol%Y203 0.059 0.050 1200 10.8
Zr02-3 mol%Ybz03 0.063 0.09
Zr02-2.9 mo1%Sc2O3 0.090 0.063
Zr02-8 mol%Y203 0.13 0.09 230 10.5
Zr02-9 mol% YzO3 0.13 0.12
Zr02-8 mol%YbZO3 0.20 0.15
Zr02-10 mol%Yb203 0.15 0.15
Zr02-8 mol%Sc203 0.30 0.12 2 70 10.7
Zr02-ll mo1%Sc203 0.30 0.30 255 10.0
Zr02-11 mol%SczO3-l wt% 0.26 0.26 250
A1203
mechanical properties as YSZ. ZrOz with 11 mol% Sc203 and 1 wt% AI2O3
appears as one of the best candidates for an intermediate temperature SOFC
because of its high oxide ion conductivity, phase stability and exceIlent
mechanical properties.
SOFC electrolytes should of course be stable under fuel conditions, such
that the electronic conductivity remains negIigible compared to the ionic
conductivity. Figure 4.8 shows the dependence of both electronic and
ionic conductivities on oxygen partial pressure for YSZ [30]. At oxygen partial
pressures of atm, the electronic conductivity can be comparable to the
ionic contribution, but this oxygen pressure is far below the normal SOFC
operating range of 0.2 1-10-20 atm.
M
0
4
0 -10 -20 -30 -40 .50
LOG OXYGEN PARTIAL PRESSURE, atrn (1,Ol x 10’ Pa)
Figure 4.8 Electrical conductivity of YSZas a function of oxygenpartialpressure.