Page 115 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 115
92 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
4.4 Ceria-Based Oxide Ion Conductors
Doped ceria has been suggested as an alternative electrolyte for low temperature
SOFCs [6, 31, 321. Reviews on the electrical conductivity and conduction
mechanism in ceria-based electrolytes have been presented by Mogensen et al.
[33] and Steele [34]. Ceria possesses the same fluorite structure as the stabilised
zirconia. Mobile oxygen vacancies are introduced by substituting Ce4+ with
trivalent rare earth ions as shown in Eq. (1). The conductivity of doped ceria
systems depends on the kind of dopant and its concentration. A typical dopant
concentration dependence of the electrical conductivity in the (CeOz)l -x(Sm203),
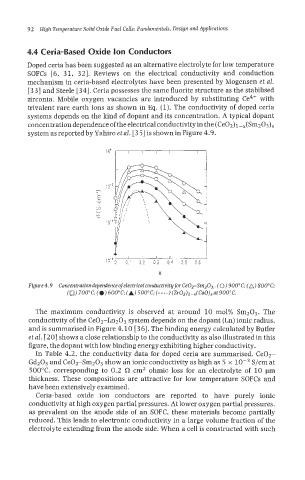
system as reported by Yahiro et al. [3 51 is shown in Figure 4.9.
X
Figure4.9 Concentrationdependenceofelectricalco~~uctivitg for CeOTSrn203.. (0) 900°C; (A) 800°C;
(0) 700"C;(~)600"C; (A) 500"C;(----)(Zr0~)~-x~CaO)xat 900°C.
The maximum conductivity is observed at around 10 mol% Sm203. The
conductivity of the Ce02-Ln203 system depends on the dopant (Ln) ionic radius,
and is summarised in Figure 4.10 [36]. The binding energy calculated by Butler
et al. [20] shows a close relationship to the conductivity as also illustrated in this
figure, the dopant with low binding energy exhibiting higher conductivity.
In Table 4.2, the conductivity data for doped ceria are summarised. Ce02-
Gd203 and Ce02-Sm203 show an ionic conductivity as high as 5 x S/cm at
500°C corresponding to 0.2 st cm2 ohmic loss for an electrolyte of 10 pm
thickness. These compositions are attractive for low temperature SOFCs and
have been extensively examined.
Ceria-based oxide ion conductors are reported to have purely ionic
conductivity at high oxygen partial pressures. At lower oxygen partial pressures,
as prevalent on the anode side of an SOFC, these materials become partially
reduced. This leads to electronic conductivity in a large volume fraction of the
electrolyte extending from the anode side. When a cell is constructed with such