Page 112 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 112
EZectroZytes 89
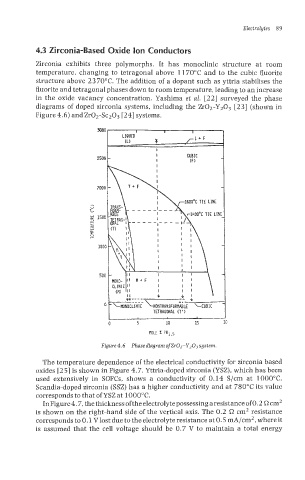
4.3 Zirconia-Based Oxide Ion Conductors
Zirconia exhibits three polymorphs. It has monoclinic structure at room
temperature, changing to tetragonal above 11 70°C and to the cubic fluorite
structure above 23 70°C. The addition of a dopant such as yttria stabilises the
fluorite and tetragonal phases down to room temperature, leading to an increase
in the oxide vacancy concentration. Yashima et al. [22] surveyed the phase
diagrams of doped zirconia systems, including the Zr02-Y203 [23] (shown in
Figure 4.6) andZr02-Sc203 [24] systems.
- I ,-L+ F
LIOUID
(L)
i
t
I
Figure 4.6 Phase diagram of ZrOZ-Y203 system.
The temperature dependence of the electrical conductivity for zirconia based
oxides [25] is shown in Figure 4.7. Yttria-doped zirconia (YSZ), which has been
used extensively in SOFCs, shows a conductivity of 0.14 S/cm at 1000°C.
Scandia-doped zirconia (SSZ) has a higher conductivity and at 780°C its value
corresponds to that ofYSZ at 1000°C.
InFigure4.7, the thickness oftheelectrolytepossessingaresistanceofO.2 Qcm2
is shown on the right-hand side of the vertical axis. The 0.2 52 cm2 resistance
corresponds to 0.1 V lost due to the electrolyte resistance at 0.5 mA/cm2, where it
is assumed that the cell voltage should be 0.7 V to maintain a total energy