Page 107 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 107
84 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
Tl°C
4
'E
0
m
--..
b
bD
0
I O~T-VK-~
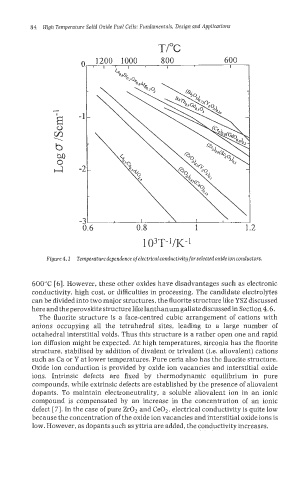
Figure 4.1 Temperature dependence of electricalconductivity for selectedoxide ion conductors.
600°C [6]. However, these other oxides have disadvantages such as electronic
conductivity, high cost, or difficulties in processing. The candidate electrolytes
can be divided into two major structures, the fluorite structure like YSZ discussed
here and the perovskite structure like lanthanum gallate discussed in Section 4.6.
The fluorite structure is a face-centred cubic arrangement of cations with
anions occupying all the tetrahedral sites, leading to a large number of
octahedral interstitial voids. Thus this structure is a rather open one and rapid
ion diffusion might be expected. At high temperatures, zirconia has the fluorite
structure, stabiIised by addition of divalent or trivalent (Le. aliovalent) cations
such as Ca or Y at lower temperatures. Pure ceria also has the fluorite structure.
Oxide ion conduction is provided by oxide ion vacancies and interstitial oxide
ions. Intrinsic defects are fixed by thermodynamic equilibrium in pure
compounds, while extrinsic defects are established by the presence of aliovalent
dopants. To maintain electroneutrality, a soluble aliovalent ion in an ionic
compound is compensated by an increase in the concentration of an ionic
defect [7]. In the case of pure ZrOz and Ce02, electrical conductivity is quite low
because the concentration of the oxide ion vacancies and interstitial oxide ions is
low. However, as dopants such as yttria are added, the conductivity increases.