Page 110 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 110
Electl-olgtes 8 7
the effect of the binding enthalpy of an associated ion can significantly affect the
population of free vacancies at low temperatures. At lower temperatures,
association is almost complete, so that
POMLel " No1 (6)
and
[Vi] = (B/T)exp(-E,/RT) ( 7)
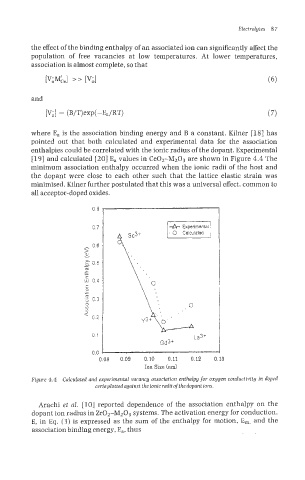
where E, is the association binding energy and B a constant. Kilner [18] has
pointed out that both calculated and experimental data for the association
enthalpies could be correlated with the ionic radius of the dopant. Experimental
[19] and calculated [20] E, values in CeO2-MzO3 are shown in Figure 4.4 The
minimum association enthalpy occurred when the ionic radii of the host and
the dopant were close to each other such that the lattice elastic strain was
minimised. Kilner further postulated that this was a universal effect, common to
all acceptor-doped oxides.
1
0.08 0.09 0.10 0.11 0.12 0.13
Ion Size hm)
Figure 4.4 Calculated and experimental vacancy association enthalpy for oxygen conductivity in doped
cerinplottedngainst theionic radiiofthedopant ions.
Arachi et al. [lo] reported dependence of the association enthalpy on the
dopant ion radius in Zr02-M203 systems. The activation energy for conduction,
E, in Eq. (3) is expressed as the sum of the enthalpy for motion, Em, and the
association binding energy, E,, thus