Page 121 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 121
98 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
1 .o
+=*F!=S==*= L3
-*--. '\ F-,
--T.x
0.8 - *$.
&-y-
e ti- ,./,:. 4
E 0.6 -
2
+ ,6';5--** _w- 1
8 -
9 0.4 - -oJ-a
E
e -
5
0.2 - * -9---o------ A-*
-.->A-
-ocO ,
/P'-
0.0 I ' I I
the unit Iattice volume of LaSc03 is similar to that of LaGa03, hole conductivity is
significant at high oxygen partial pressures. However, oxide ion conductivity
in all reported perovskite oxides is lower than that of YSZ, since solid solubility of
the dopant in these oxides is limited.
In AB03 perovskites, it was originally believed that the electric or dielectric
properties were strongly dependent on B site cations. However, migrating oxide
ions have to pass through the triangular space consisting of two Iarge A and one
small B site cations in the crystal lattice. Theoretical calculations now suggest
that the enlargement in size of this triangular space is important for improving
the migration of oxide ions in the crystal lattice [58]. Therefore, the ionic size
of the A site cation is also significant in determining the oxide ion conductivity.
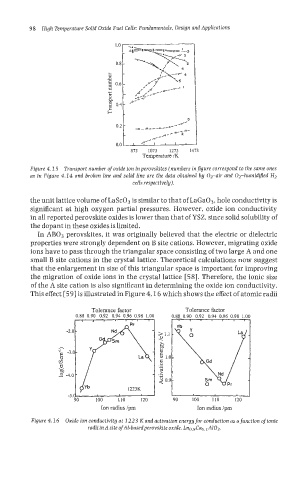
This effect [59] is illustrated in Figure 4.1 6 which shows the effect of atomic radii
-
h
'E -3.0-
. E 1.0-
u
v)
0
v - .- d
m
rj
*
2 -4.0- .I
p 0.9- Sm
Yb 1223K Pr
-5.0
Figure 4.1 6 Oxide ion conductivity at 1223 K and activation energy for conduction as a function of ionic
radiiinA site ofAl-basedperovskite oxide, Lno.9Cao.IA103.