Page 122 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 122
Electrolytes 99
of A site cations on the oxide ion conductivity and its apparent activation energy
in Lno.9Cao.lA103. It is clear that the ionic radii of A site cations, dictating the
size of the triangular space, have a strong affect on the mobility of oxide ions.
Oxide ion conductivity and activation energy increase and decrease respectively
with increasing ionic radius, and PrA103 exhibits the highest conductivity
among the Al-based perovskites. These results suggest that enlargement of the
size of the triangular space in the lattice can be used to increase the ionic
conduction. From these results, higher ion conductivity is to be expected in
perovskites with larger unit lattice dimensions [SO].
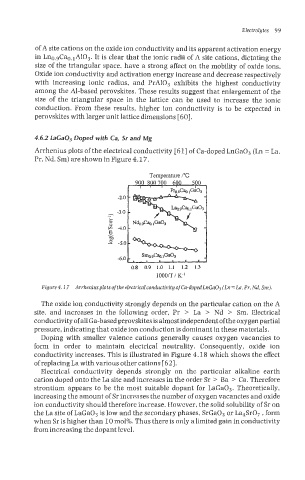
4.6.2 LaCaOs Doped with Ca, Sr and Mg
Arrhenius plots of the electrical conductivity [61] of Ca-doped LnGa03 (Ln = La,
Pr, Nd, Sm) are shown in Figure 4.1 7.
2 -5.0 - L
v
tJl
-6,0 S%.9C%.IGa03 ' 1
1
Figure4.17 Arrheniusplots oftheelectricalconductivity of Ca-doped LnGaOs (Ln = La, Pr, Ed, Sm).
The oxide ion conductivity strongly depends on the particular cation on the A
site, and increases in the following order, Pr > La > Nd > Sm. Electrical
conductivity of all Ga-based perovskites is almost independent of the oxygen partial
pressure, indicating that oxide ion conduction is dominant in these materials.
Doping with smaller valence cations generally causes oxygen vacancies to
form in order to maintain electrical neutrality. Consequently, oxide ion
conductivity increases. This is illustrated in Figure 4.18 which shows the effect
of replacing La with various other cations [62].
Electrical conductivity depends strongly on the particular alkaline earth
cation doped onto the La site and increases in the order Sr > Ba > Ca. Therefore
strontium appears to be the most suitable dopant for LaGa03. Theoretically,
increasing the amount of Sr increases the number of oxygen vacancies and oxide
ion conductivity should therefore increase. However, the solid solubility of Sr on
the La site of LaGa03 is low and the secondary phases, SrGa03 or La4Sr07 , form
when Sr is higher than 10 mol%. Thus there is only a limited gain in conductivity
from increasing the dopant level.