Page 127 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 127
104 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
reducing conditions, Yamaji et al. [83] found by SIMS analysis that the Ga
content decreased at the surface of LSGM owing to the high vapour pressure of
GaO. However, the vapour pressure of GaO decreases exponentially with
decreasing temperature and is negligible at 600°C. Therefore, evaporation of
GaO does not appear to be a problem when LSGM is used as an electrolyte in
SOPCs at intermediate temperatures.
Hayashi et al. [84] and Ishihara et al. [79] investigated thermal expansion of
LSGM and showed that it increases with increasing dopant content. The
estimated average thermal expansion coefficient was around 11.5 x 10-6/1< in
the temperature range from room temperature to 1000°C. This is slightly larger
than that of YSZ but slightly smaller than that of than CGO.
The diffusivity of oxide ions in LSGM was studied with l80 tracer diffusion
measurements [85]. LSGM exhibits a large diffusion coefficient because of the
larger mobility of oxide ions compared to that in the fluorite structured oxides
(Table 4.4). The perovskite structure has a large free volume in its lattice and this
gives a high diffusivity of oxide ions, resulting in high conductivity.
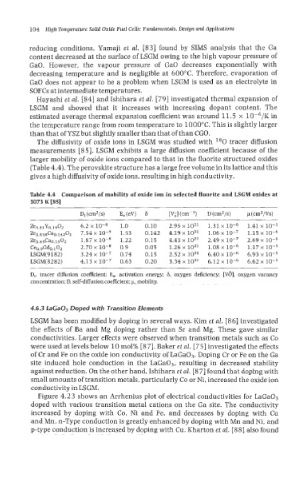
Table 4.4 Comparison of mobility of oxide ion in selected fluorite and LSGM oxides at
1073 I< [85]
Dt(cm2/s) E,(eV) 6 [Vi] (~rn-~) D (cm2/s) p (cm2/Vs)
Zro.s1Yo.190z 6.2 x 1.0 0.10 2.95 x 10’’ 1.31 x 1.41 x 10-j
Zro.8j8Cao.14202 7.54 x 1.53 0.142 4.19 x 1021 1.06 x 1.15 x
Zro.s5Cao.1502 1.87 x lo-* 1.22 0.15 4.43 x lo2’ 2.49 x 2.69 x
Ce0.9Gd0.102 2.70 x lo-* 0.9 0.05 1.26 x lo2’ 1.08 x 1.17 x
LSGM(9182) 3.24 x lo-’ 0.74 0.15 2.52 x 1021 6.40 x 6.93 x
LSGM( 8 2 82) 4.13 x lo-’ 0.63 0.20 3.34 x loz1 6.12 x 6.62 x
D,, tracer diffusion coefficient; E,, activation energy; 6, oxygen deficiency; [VO], oxygen vacancy
concentration; D, self-diffusion coefficient; p, mobility.
4.63 LaGaO, Doped with Transition Elements
LSGM has been modified by doping in several ways. Kim et al. [86] investigated
the effects of Ba and Mg doping rather than Sr and Mg. These gave similar
conductivities. Larger effects were observed when transition metals such as Co
were used at levels below 10 mol% 1871. Baker et al. [75] investigated the effects
of Cr and Fe on the oxide ion conductivity of LaGa03. Doping Cr or Fe on the Ga
site induced hole conduction in the LaGa03, resulting in decreased stability
against reduction. On the other hand, Ishihara et aI. [8 71 found that doping with
small amounts of transition metals, particularly Co or Ni, increased the oxide ion
conductivity in LSGM.
Figure 4.23 shows an Arrhenius plot of electrical conductivities for LaGa03
doped with various transition metal cations on the Ga site. The conductivity
increased by doping with Co, Ni and Fe, and decreases by doping with Cu
and Mn. n-Type conduction is greatly enhanced by doping with Mn and Ni, and
p-type conduction is increased by doping with Cu. Kharton et al. [88] also found