Page 132 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 132
EZectroZytes 109
Temperature PC
800 400 200 100 50
3 f
I , BaTh0.9Gd0.103
2
-3
-4
-5
1 1.5 2 2.5 3 3.5
I OOO/T/K-i
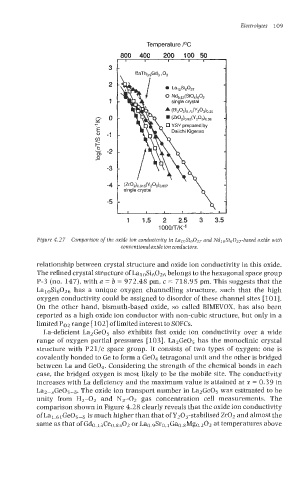
Figure 4.27 Comparison of the oxide ion conductivity in La10Si6027 and Xd~oSi60~~-based oxide with
conventional oxide ion conductors.
relationship between crystal structure and oxide ion conductivity in this oxide.
The refined crystal structure of LalOSi6OZ6 belongs to the hexagonal space group
P-3 (no. 147), with a = b = 972.48 pm, c = 718.95 pm. This suggests that the
LalOSi6Oz6 has a unique oxygen channelIing structure, such that the high
oxygen conductivity could be assigned to disorder of these channel sites [loll.
On the other hand, bismuth-based oxide, so called BIMEVOX, has also been
reported as a high oxide ion conductor with non-cubic structure, but only in a
limited PO2 range [ 1021 of limited interest to SOFCs.
La-deficient La2GeOj also exhibits fast oxide ion conductivity over a wide
range of oxygen partial pressures [103]. La2Ge05 has the monoclinic crystal
structure with P21/c space group. It consists of two types of oxygen; one is
covalently bonded to Ge to form a Ge04 tetragonal unit and the other is bridged
between La and Ge04. Considering the strength of the chemical bonds in each
case, the bridged oxygen is most likely to be the mobile site. The conductivity
increases with La deficiency and the maximum value is attained at x = 0.39 in
La2-,GeOs-~. The oxide ion transport number in LaZGeO5 was estimated to be
unity from Hz-O2 and Nz-02 gas concentration cell measurements. The
comparison shown in Figure 4.28 clearly reveals that the oxide ion conductivity
of Lal,61Ge05-s is much higher than that of Y203-stabilised Zr02 and almost the
same as that of Gdo.lsCe0.as02 or Lao.9Sr0.1Gao.8Mgo.20~ at temperatures above