Page 130 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 130
Electrolytes 107
high compared with those of fI uorite-structured oxides. Among these, Ba21n205
is attracting much interest from the viewpoint of SOFC electrolyte.
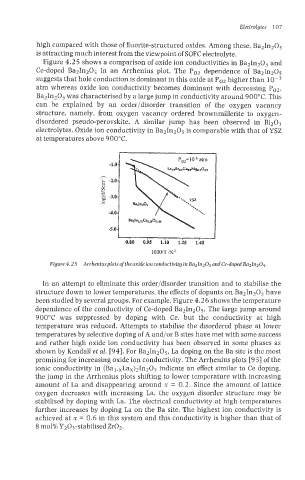
Figure 4.2 5 shows a comparison of oxide ion conductivities in Ba21n20S and
Ce-doped Ba&1~0~ in an Arrhenius plot. The PO2 dependence of Ba21n205
suggests that hole conduction is dominant in this oxide at PO2 higher than
atm whereas oxide ion conductivity becomes dominant with decreasing PO2.
Ba21n205 was characterised by a large jump in conductivity around 900°C. This
can be explained by an order/disorder transition of the oxygen vacancy
structure, namely, from oxygen vacancy ordered brownmillerite to oxygen-
disordered pseudo-perovskite. A similar jump has been observed in Bi2O3
electrolytes. Oxide ion conductivity in Ba21n20 is comparable with that of YSZ
at temperatures above 900°C.
~nIS~~GawMtb.1019
-... YSZ
d
4
-5.0-
0.80 0.95 t10 1.25 1.40
1OOOm /IC-'
Figure 4.25 Arrheniusplots of the oxide ion conductivity in Ba21n205 and Ce-doped Ba21n205.
In an attempt to eliminate this order/disorder transition and to stabilise the
structure down to lower temperatures, the effects of dopants on Ba21n20j have
been studied by several groups. For example, Figure 4.26 shows the temperature
dependence of the conductivity of Ce-doped Ba21n205. The large jump around
900°C was suppressed by doping with Ce, but the conductivity at high
temperature was reduced. Attempts to stabilise the disordered phase at lower
temperatures by selective doping of A and/or B sites have met with some success
and rather high oxide ion conductivity has been observed in some phases as
shown by Kendall et al. [94]. For Ba21n205, La doping on the Ba site is the most
promising for increasing oxide ion conductivity. The Arrhenius plots [9 51 of the
ionic conductivity in (Bal..xLax)21n205 indicate an effect similar to Ce doping,
the jump in the Arrhenius plots shifting to lower temperature with increasing
amount of La and disappearing around x = 0.2. Since the amount of lattice
oxygen decreases with increasing La, the oxygen disorder structure may be
stabilised by doping with La. The electrical conductivity at high temperatures
further increases by doping La on the Ba site. The highest ion conductivity is
achieved at x = 0.6 in this system and this conductivity is higher than that of
8 mol% Y203-stabilised Zr02.