Page 123 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 123
100 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
I l l
0 tsosSro.1GaQ3
-1s - p %.&.lo.iGaOs
0 LPa.SBPo.lGaP3
," -2.5 "
B
CC
-6
-
v
%I
-3.5 -
-4.5 - ' . '. ' . ' . ' .
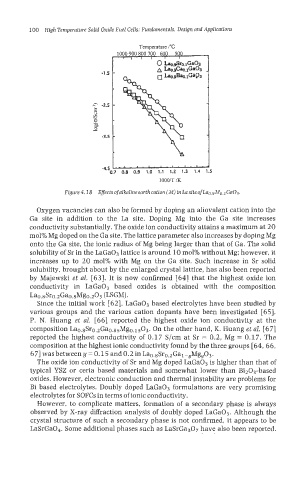
Oxygen vacancies can also be formed by doping an aliovalent cation into the
Ga site in addition to the La site. Doping Mg into the Ga site increases
conductivity substantially. The oxide ion conductivity attains a maximum at 20
mol% Mg doped on the Ga site. The lattice parameter also increases by doping Mg
onto the Ga site, the ionic radius of Mg being larger than that of Ga. The solid
solubility of Sr in the LaGa03 lattice is around 10 mol% without Mg; however, it
increases up to 20 mol% with Mg on the Ga site. Such increase in Sr solid
solubility, brought about by the enlarged crystal lattice, has also been reported
by Majewski et al. [63]. It is now confirmed [64] that the highest oxide ion
conductivity in LaGaO3 based oxides is obtained with the composition
La0.8Sr0.2Ga0.8Mg0.203 (LSGM).
Since the initial work [62], LaGa03 based electrolytes have been studied by
various groups and the various cation dopants have been investigated [65].
P. N. Huang et al. [66] reported the highest oxide ion conductivity at the
composition Lao.8Sro.zGao.8sMgo.~~03. On the other hand, I<. Huang et al. [67]
reported the highest conductivity of 0.17 S/cm at Sr = 0.2, Mg = 0.17. The
composition at the highest ionic conductivity found by the three groups [64, 66,
671 was betweeny = 0.15 and 0.2 inLao.8Sro.2Gal_,Mg,03.
The oxide ion conductivity of Sr and Mg doped LaGa03 is higher than that of
typical YSZ or ceria based materials and somewhat lower than Bi203-based
oxides. However, electronic conduction and thermal instability are problems for
Bi based electrolytes. Doubly doped LaGa03 formulations are very promising
electrolytes for SOFCs in terms of ionic conductivity.
However, to complicate matters, formation of a secondary phase is always
observed by X-ray diffraction analysis of doubly doped LaGa03. Although the
crystal structure of such a secondary phase is not confirmed, it appears to be
LaSrGa04. Some additional phases such as LaSrGa307 have also been reported.