Page 25 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 25
6 High Temperature Solid Oxide Fuel Cells: Fundamentak, Design and Applications
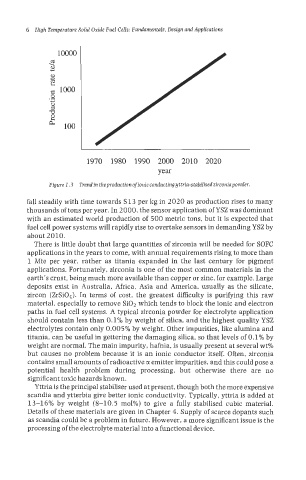
1970 1980 1990 2000 2010 2020
year
Figure 1.3 Trend in theproduction ofionic conducting yttrin-stabilisedzirconinpowder.
fall steadily with time towards $13 per kg in 2020 as production rises to many
thousands of tons per year. In 2000, the sensor application of YSZ was dominant
with an estimated world production of 500 metric tons, but it is expected that
fuel cell power systems will rapidly rise to overtake sensors in demanding YSZ by
about 2 0 10,
There is little doubt that large quantities of zirconia will be needed for SOFC
applications in the years to come, with annual requirements rising to more than
1 Mte per year, rather as titania expanded in the last century for pigment
applications. Fortunately, zirconia is one of the most common materials in the
earth’s crust, being much more available than copper or zinc, for example. Large
deposits exist in Australia, Africa, Asia and America, usually as the silicate,
zircon (ZrSi04). In terms of cost, the greatest difficulty is purifying this raw
material, especially to remove SiOz which tends to block the ionic and electron
paths in fuel cell systems. A typical zirconia powder for electrolyte application
should contain less than 0.1% by weight of silica, and the highest quality YSZ
electrolytes contain only 0.005% by weight. Other impurities, like alumina and
titania, can be useful in gettering the damaging silica, so that levels of 0.1% by
weight are normal. The main impurity, hafnia, is usually present at several wt%
but causes no problem because it is an ionic conductor itself. Often, zirconia
contains small amounts of radioactive a emitter impurities, and this could pose a
potential health problem during processing, but otherwise there are no
significant toxic hazards known.
Yttria is the principal stabiliser used at present, though both the more expensive
scandia and ytterbia give better ionic conductivity. Typically, yttria is added at
13-16% by weight (8-10.5 mol%) to give a fully stabilised cubic material.
Details of these materials are given in Chapter 4. Supply of scarce dopants such
as scandia could be a problem in future. However, a more significant issue is the
processing of the electrolyte material into a functional device.