Page 30 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 30
Introduction to SOFCs 11
produced at one time. One such technique was developed by Minh to tape
calender the two electrodes and the electrolyte together as one sheet [22].
Another method, that of co-extrusion, was developed to make the anode and
electrolyte in one step [2 31.
1.7 interconnection for Electrically Connecting the Cells
The interconnection requires two interconnect wires but these are often
combined into a single material which makes contact with the anode on one side
and the cathode on the other.
Ideally an inert and impervious conducting material is needed to withstand
both the oxidising potential on the air electrode and the reducing condition at
the fuel side as described in Chapter 7. In the SOFC, since 1974, lanthanum
chromite has been used to carry out this function for the systems operating near
1000°C. This material has almost exactly the same thermal expansion coefficient
as YSZ, depending on doping. Typically strontium dopant has been used at 20
mol% to give an expansion coefficient of about 11 x 1W6/K. For systems
operating at lower temperatures, 700-8 50°C, it is conceivable that metallic
alloys like ferritic stainless steel could be used. Other chromium-based alloys
have also been tested [24].
Magnesium-doped lanthanum chromite has been the material most used by
Westinghouse (now Siemens Westinghouse) to produce single cells and stacks
of their tubular design [ll]. The material was initially deposited by an
electrochemical vapour deposition process to form a strip along the lanthanum
manganite tube and is now deposited by plasma spraying. This made contact
with the anode of the neighbouring cell to give series connection along a stack as
shown in Chapter 8. This material has worked very well and has provided single
cell lifetimes of up to 70,000 h in hydrogen.
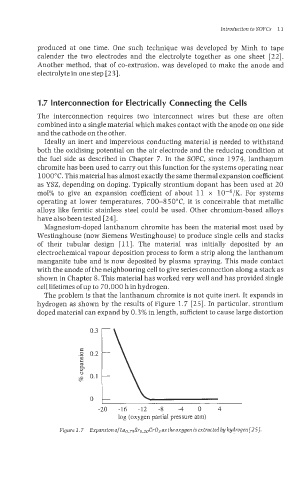
The problem is that the lanthanum chromite is not quite inert. It expands in
hydrogen as shown by the resuIts of Figure 1.7 [25]. In particular, strontium
doped material can expand by 0.3% in length, sufficient to cause large distortion
-
0.3
c -
.g 0.2
%
F:
0
&? 0.1 -
0 -