Page 29 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 29
10 High Ternpercrtrrrc, Solid Orirle Fuel Cells: Fundarncntals, Design and Applications
19 73 by lanthanum manganites. Typically, Lao.sSro.2Mn03 (LSM) gives a good
combination of electronic conductivity and expansion coefficient matching, and
is now available commercially for SOFC applications. Higher conductivity can be
obtained at higher dopant levels, but the expansion coefficient then becomes too
high. Lanthanum cobaltite is a much better material from the catalytic and
conduction standpoints but is too reactive with zirconia and also expands too
much. Even the manganite reacts with zirconia above 1400°C and produces an
insulating layer of lanthanum zirconate which increases the resistance
enormously. Therefore, firing of the cathode materials on YSZ tends to be kept
below 1 300"C, and a minor excess of manganese is used to inhibit the reaction.
The manganese can be seen diffusing into the YSZ at high temperatures, a
blackened region gradually penetrating the normally white electrolyte.
In order to minimise the resistance at the LSM cathode, especially as the
operating temperature of the SOFC is reduced below lOOO"C, it has become
normal practice to mix the LSM powder with YSZ powder, roughly in 50/50
proportion, to form the first layer of cathode material at the electrolyte surface.
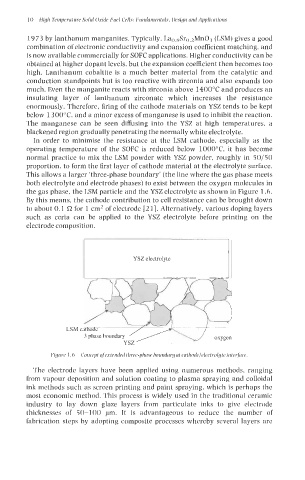
This allows a larger 'three-phase boundary' (the line where the gas phase meets
both electrolyte and electrode phases) to exist between the oxygen molecules in
the gas phase, the LSM particle and the YSZ electrolyte as shown in Figure 1.6.
By this means, the cathode contribution to cell resistance can be brought down
to about 0.1 C2 for 1 cm2 of electrode [21]. Alternatively, various doping layers
such as ceria can be applied to the YSZ electrolyte before printing on the
electrode composition.
.
LSM cathode
3 phase boundary oxygen
Figurr 1 .h Concrpt ofrrtrndrd thrrr-phase houndnry at cnthodelelectrolgte interJace.
The electrode layers have been applied using numerous methods, ranging
from vapour deposition and solution coating to plasma spraying and colloidal
ink methods such as screen printing and paint spraying, which is perhaps the
most economic method. This process is widely used in the traditional ceramic
industry to lay down glaze layers from particulate inks to give electrode
thicknesses of 50-100 pm. It is advantageous to reduce the number of
fabrication steps by adopting composite processes whereby several layers are