Page 293 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 293
2 70 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
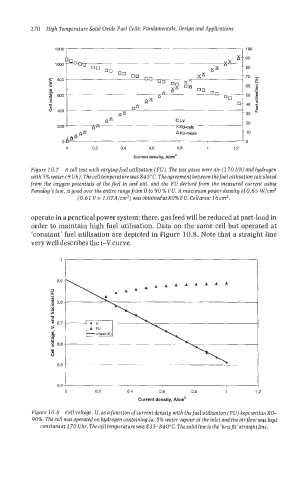
90
70
400
20
200 x FUalc
A FU-meas 10
0 0.2 0-4 0,6 0,8 1 12
current density, ~/cm~
Figure 10.7 A cell test with varying fuel utilisation (FU). The test gases were air (7 70 l/h) and hydrogen
with 5% water (9 l/h), The cell temperature was 845°C. Theagreement between thefuel utilisation calculated
from the oxggen potentials of the fuel in and out. and the FU derived from the measured current using
Faraday’s law, is good over the entire rangefrom 0 to 90 % FU. A maximum power density ofO.65 W/cm2
(0.62 Vx 2.07A/cm2) wasobtainedatSO%FU. Cellarea: 76cm2.
operate in a practical power system: there, gas feed will be reduced at part-load in
order to maintain high fuel utilisation. Data on the same cell but operated at
‘constant’ fuel utilisation are depicted in Figure 10.8. Note that a straight line
very well describes the i-V curve.
1,
0 02 0.4 0,6 0-8 1 12
Current density, Ncm2
Figure 10.8 Cell voltage, U, as a function of current density with the fuel utiiisation (FU) kept within 80-
90%. The cell was operatedon hydrogen containing ca. 5% water vapour at the inlet and the airflow was kept
constant at 1701/hr. Thecelltemperature was835-840°C. Thesolidlineis the ‘bestfit’straightline.