Page 309 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 309
Testing of EZectrodes, Cells and Short Stacks 285
0 20 40 60 80
%H,O in feed
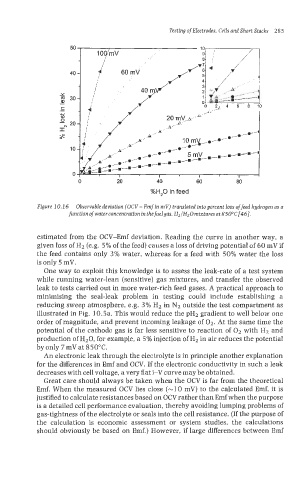
Figure 10.1 6 Observable deviation (OCV - Emf in mV) translated into percent loss of feed hydrogen as a
function ofwaterconcentration in the fuelgas, H2/H20mixturesnt 850°C[46].
estimated from the OCV-Emf deviation. Reading the curve in another way, a
given loss of H2 (e.g. 5% of the feed) causes a loss of driving potential of 60 mV if
the feed contains only 3% water, whereas for a feed with 50% water the loss
is only 5 mV.
One way to exploit this knowledge is to assess the leak-rate of a test system
while running water-lean (sensitive) gas mixtures, and transfer the observed
leak to tests carried out in more water-rich feed gases. A practical approach to
minimising the seal-leak problem in testing could include establishing a
reducing sweep atmosphere, e.g. 3% H2 in N2 outside the test compartment as
illustrated in Fig. 10.5a. This would reduce the pH2 gradient to well below one
order of magnitude, and prevent incoming leakage of 02. At the same time the
potential of the cathode gas is far less sensitive to reaction of O2 with H2 and
production of H20, for example, a 5% injection of H2 in air reduces the potential
by only 7 mV at 850°C.
An electronic leak through the electrolyte is in principle another explanation
for the differences in Emf and OCV. If the electronic conductivity in such a leak
decreases with cell voltage, a very flat i-V curve may be obtained.
Great care should always be taken when the OCV is far from the theoretical
Emf. When the measured OCV lies close (-10 mV) to the calculated Emf, it is
justified to calculate resistances based on OCV rather than Emf when the purpose
is a detailed celI performance evaluation, thereby avoiding lumping problems of
gas-tightness of the electrolyte or seals into the cell resistance. (If the purpose of
the calculation is economic assessment or system studies, the calculations
should obviously be based on Emf.) However, if large differences between Emf