Page 51 - Illustrated Pocket Dictionary of Chromatography
P. 51
46 CROWN ETHERS
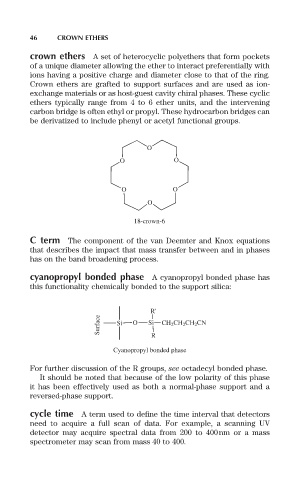
crown ethers A set of heterocyclic polyethers that form pockets
of a unique diameter allowing the ether to interact preferentially with
ions having a positive charge and diameter close to that of the ring.
Crown ethers are grafted to support surfaces and are used as ion-
exchange materials or as host-guest cavity chiral phases. These cyclic
ethers typically range from 4 to 6 ether units, and the intervening
carbon bridge is often ethyl or propyl. These hydrocarbon bridges can
be derivatized to include phenyl or acetyl functional groups.
O
O O
O O
O
18-crown-6
C term The component of the van Deemter and Knox equations
that describes the impact that mass transfer between and in phases
has on the band broadening process.
cyanopropyl bonded phase A cyanopropyl bonded phase has
this functionality chemically bonded to the support silica:
R'
Surface Si O Si CH 2 CH 2 CH 2 CN
R
Cyanopropyl bonded phase
For further discussion of the R groups, see octadecyl bonded phase.
It should be noted that because of the low polarity of this phase
it has been effectively used as both a normal-phase support and a
reversed-phase support.
cycle time A term used to define the time interval that detectors
need to acquire a full scan of data. For example, a scanning UV
detector may acquire spectral data from 200 to 400nm or a mass
spectrometer may scan from mass 40 to 400.