Page 136 - Industrial Wastewater Treatment, Recycling and Reuse
P. 136
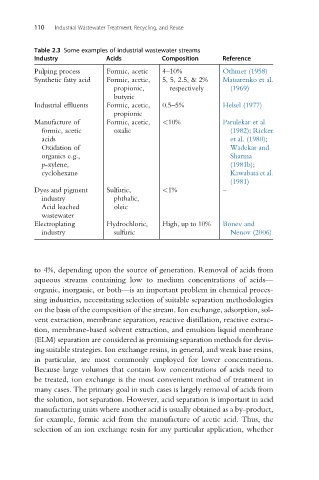
110 Industrial Wastewater Treatment, Recycling, and Reuse
Table 2.3 Some examples of industrial wastewater streams
Industry Acids Composition Reference
Pulping process Formic, acetic 4–10% Othmer (1958)
Synthetic fatty acid Formic, acetic, 5, 5, 2.5, & 2% Matsarenko et al.
propionic, respectively (1969)
butyric
Industrial effluents Formic, acetic, 0.5–5% Helsel (1977)
propionic
Manufacture of Formic, acetic, <10% Parulekar et al.
formic, acetic oxalic (1982); Ricker
acids et al. (1980);
Oxidation of Wadekar and
organics e.g., Sharma
p-xylene, (1981b);
cyclohexane Kawabata et al.
(1981)
Dyes and pigment Sulfuric, <1% –
industry phthalic,
Acid leached oleic
wastewater
Electroplating Hydrochloric, High, up to 10% Bonev and
industry sulfuric Nenov (2006)
to 4%, depending upon the source of generation. Removal of acids from
aqueous streams containing low to medium concentrations of acids—
organic, inorganic, or both—is an important problem in chemical proces-
sing industries, necessitating selection of suitable separation methodologies
on the basis of the composition of the stream. Ion exchange, adsorption, sol-
vent extraction, membrane separation, reactive distillation, reactive extrac-
tion, membrane-based solvent extraction, and emulsion liquid membrane
(ELM) separation are considered as promising separation methods for devis-
ing suitable strategies. Ion exchange resins, in general, and weak base resins,
in particular, are most commonly employed for lower concentrations.
Because large volumes that contain low concentrations of acids need to
be treated, ion exchange is the most convenient method of treatment in
many cases. The primary goal in such cases is largely removal of acids from
the solution, not separation. However, acid separation is important in acid
manufacturing units where another acid is usually obtained as a by-product,
for example, formic acid from the manufacture of acetic acid. Thus, the
selection of an ion exchange resin for any particular application, whether