Page 244 - Industrial Wastewater Treatment, Recycling and Reuse
P. 244
218 Industrial Wastewater Treatment, Recycling, and Reuse
Feed
Chemicals
(catalyst, acid)
Operating
conditions:
T: 120 -200 °C
p: 3- 20 bar
t <3 h
Heating
(if required)
Off-gas
O 2
Treated
wastewater
R
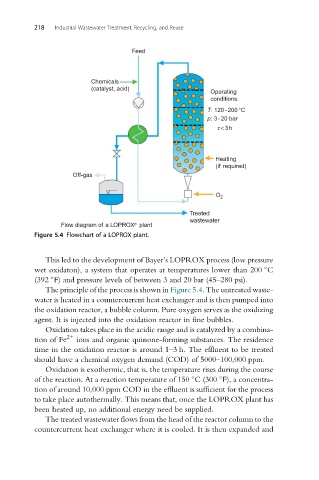
Flow diagram of a LOPROX plant
Figure 5.4 Flowchart of a LOPROX plant.
This led to the development of Bayer’s LOPROX process (low pressure
wet oxidaton), a system that operates at temperatures lower than 200 C
(392 F) and pressure levels of between 3 and 20 bar (45–280 psi).
The principle of the process is shown in Figure 5.4. The untreated waste-
water is heated in a countercurrent heat exchanger and is then pumped into
the oxidation reactor, a bubble column. Pure oxygen serves as the oxidizing
agent. It is injected into the oxidation reactor in fine bubbles.
Oxidation takes place in the acidic range and is catalyzed by a combina-
tion of Fe 2+ ions and organic quinone-forming substances. The residence
time in the oxidation reactor is around 1–3 h. The effluent to be treated
should have a chemical oxygen demand (COD) of 5000–100,000 ppm.
Oxidation is exothermic, that is, the temperature rises during the course
of the reaction. At a reaction temperature of 150 C (300 F), a concentra-
tion of around 10,000 ppm COD in the effluent is sufficient for the process
to take place autothermally. This means that, once the LOPROX plant has
been heated up, no additional energy need be supplied.
The treated wastewater flows from the head of the reactor column to the
countercurrent heat exchanger where it is cooled. It is then expanded and