Page 245 - Industrial Wastewater Treatment, Recycling and Reuse
P. 245
Novel Technologies for the Elimination of Pollutants 219
transferred to a biological treatment plant. This generates a small quantity of
flue gas containing CO 2 and small part of CO, which is then treated in a
separate process.
Depending on the reaction and the type of organic compound being
treated, 60–90% of the organic carbon is converted to CO 2 during wet oxi-
dation. What remains in the wastewater generally consists of easily biode-
gradable fragments of the aromatic core (acetic acid, acetone, etc.). If the
substituting groups include S, CI, or P, then H 2 SO 4 , HCl, or H 3 PO 4 are
respectively formed. Most of the organic nitrogen is converted to ammonia,
which can be removed from the wastewater by stripping or in the biological
plant by nitrification/dinitrification.
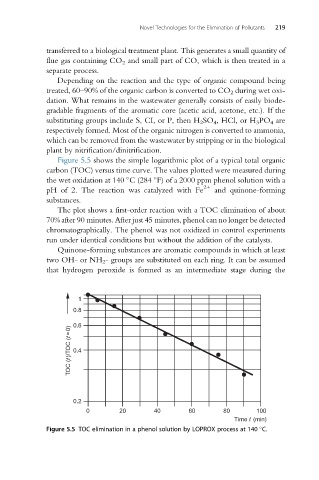
Figure 5.5 shows the simple logarithmic plot of a typical total organic
carbon (TOC) versus time curve. The values plotted were measured during
the wet oxidation at 140 C (284 F) of a 2000 ppm phenol solution with a
2+
pH of 2. The reaction was catalyzed with Fe and quinone-forming
substances.
The plot shows a first-order reaction with a TOC elimination of about
70% after 90 minutes. After just 45 minutes, phenol can no longer be detected
chromatographically. The phenol was not oxidized in control experiments
run under identical conditions but without the addition of the catalysts.
Quinone-forming substances are aromatic compounds in which at least
two OH- or NH 2 - groups are substituted on each ring. It can be assumed
that hydrogen peroxide is formed as an intermediate stage during the
1
0.8
0.6
TOC (t )/TOC (t =0) 0.4
0.2
0 20 40 60 80 100
Time t (min)
Figure 5.5 TOC elimination in a phenol solution by LOPROX process at 140 C.