Page 246 - Industrial Wastewater Treatment, Recycling and Reuse
P. 246
220 Industrial Wastewater Treatment, Recycling, and Reuse
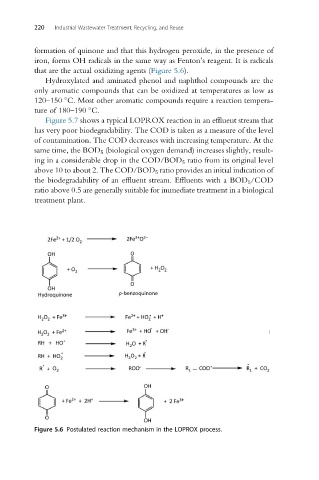
formation of quinone and that this hydrogen peroxide, in the presence of
iron, forms OH radicals in the same way as Fenton’s reagent. It is radicals
that are the actual oxidizing agents (Figure 5.6).
Hydroxylated and aminated phenol and naphthol compounds are the
only aromatic compounds that can be oxidized at temperatures as low as
120–150 C. Most other aromatic compounds require a reaction tempera-
ture of 180–190 C.
Figure 5.7 shows a typical LOPROX reaction in an effluent stream that
has very poor biodegradability. The COD is taken as a measure of the level
of contamination. The COD decreases with increasing temperature. At the
same time, the BOD 5 (biological oxygen demand) increases slightly, result-
ing in a considerable drop in the COD/BOD 5 ratio from its original level
above 10 to about 2. The COD/BOD 5 ratio provides an initial indication of
the biodegradability of an effluent stream. Effluents with a BOD 5 /COD
ratio above 0.5 are generally suitable for immediate treatment in a biological
treatment plant.
Figure 5.6 Postulated reaction mechanism in the LOPROX process.