Page 197 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 197
Secondary Ion Mass Spectrometry 183
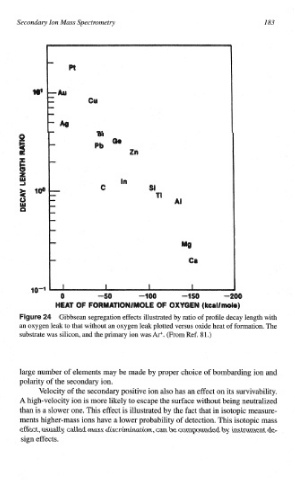
ur Gibbsean segregation effects illustrated by ratio of profile decay length with
an oxygen leak to that without an oxygen leak plotted versus oxide heat of formation. The
substrate was silicon, and the primary ion was Ar+. (From Ref. 81.)
large number of elements may be made by proper choice of bombarding ion and
polarity of the secondary ion.
Velocity of the secondary positive ion also has an effect on its su~ivabi~ity.
A high-velocity ion is more likely to escape the surface without being neutralized
than is a slower one. This effect is illus~ated by the fact that in isotopic measure-
ments higher-mass ions have a lower probability of detection. This isotopic mass
effect, usually called muss ~iscriminutiun, can be compounded by inst~ment de-
sign effects.