Page 200 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 200
I86 Cristy
4
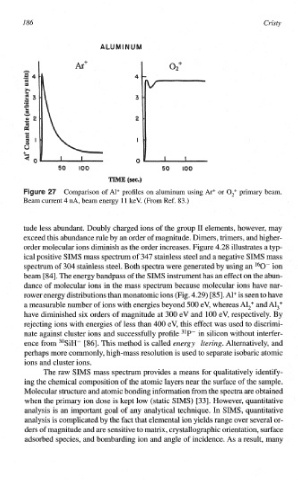
Comparison of Al+ profiles on aluminum using Ar+ or O,+ primary beam.
Beam current 4 nA, beam energy l l keV. (From Ref. 83.)
tude less abundant. Doubly charged ions of the group I1 elements, however, may
exceed this abundance rule by an order of magnitude. Dimers, trimers, and higher-
order molecular ions diminish as the order increases. Figure 4.28 illustrates a typ-
of
ical positive SIMS mass spectrum 347 stainless steel and a negative SIMS mass
an l60" ion
spect~m of 304 stainless steel. Both spectra were generated by using
beam [84]. The energy bandpass of the SIMS instrument has an effect on the abun-
dance of molecular ions in the mass spectrum because molecular ions have nar-
is
rower energy dis~butions than monatomic ions (Fig. 4.29) [85]. Al+ seen to have
a measurable number of ions with energies beyond 500 eV, whereas A12+ and AI,+
have dimini~hed six orders of magnitude at 300 eV and 100 eV, respectively. By
rejecting ions with energies of less than 400 eV, this effect was used to discrimi-
nate against cluster ions and successfully profile 31P- in silicon without interfer-
ence from 30SiH" [84]. This method is called energy ltering. Alternatively, and
perhaps more conxnonly, high-mass resolution used to separate isobaric atomic
is
ions and cluster ions.
The raw SMS mass spectrum provides a means for qualitatively identify-
ing the chemical composition of the atomic layers near the surface of the sample.
Molecular structure and atomic bonding information from spectra are obtained
the
when the primary ion dose is kept low (static SIMS) [33]. However, quantitative
analysis is an important goal of any analytical technique. In SIMS, quantitative
analysis is complicated by the fact that elemental ion yields range over several or-
ders of magnitude and are sensitive matrix, c~stallographic orientation, surface
to
adsorbed species, and omb bar ding ion and angle of incidence. As a result, many