Page 189 - Instant notes
P. 189
Energetics and mechanisms 175
from which is obtained the pre-exponential Arrhenius factor, . The
experimental value of A is often smaller than the calculated value because molecules
must also have specific orientation to each other at the moment of collision as well as
sufficient kinetic energy. This is accounted for by including a steric factor, P, in the pre-
exponential factor, , to represent the probability that an encounter has the
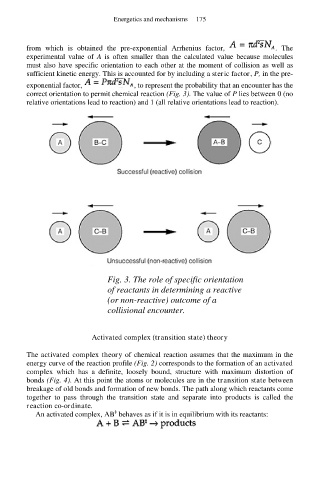
correct orientation to permit chemical reaction (Fig. 3). The value of P lies between 0 (no
relative orientations lead to reaction) and 1 (all relative orientations lead to reaction).
Fig. 3. The role of specific orientation
of reactants in determining a reactive
(or non-reactive) outcome of a
collisional encounter.
Activated complex (transition state) theory
The activated complex theory of chemical reaction assumes that the maximum in the
energy curve of the reaction profile (Fig. 2) corresponds to the formation of an activated
complex which has a definite, loosely bound, structure with maximum distortion of
bonds (Fig. 4). At this point the atoms or molecules are in the transition state between
breakage of old bonds and formation of new bonds. The path along which reactants come
together to pass through the transition state and separate into products is called the
reaction co-ordinate.
‡
An activated complex, AB behaves as if it is in equilibrium with its reactants: