Page 190 - Instant notes
P. 190
Physical chemistry 176
with concentration described in the normal way (neglecting activity coefficients) by an
‡
equilibrium constant, K , (Topic C1):
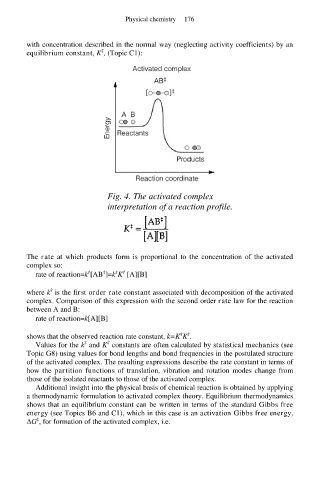
Fig. 4. The activated complex
interpretation of a reaction profile.
The rate at which products form is proportional to the concentration of the activated
complex so:
‡
‡
‡
‡
rate of reaction=k [AB ]=k K [A][B]
‡
where k is the first order rate constant associated with decomposition of the activated
complex. Comparison of this expression with the second order rate law for the reaction
between A and B:
rate of reaction=k[A][B]
‡
‡
shows that the observed reaction rate constant, k=K K .
‡
‡
Values for the k and K constants are often calculated by statistical mechanics (see
Topic G8) using values for bond lengths and bond frequencies in the postulated structure
of the activated complex. The resulting expressions describe the rate constant in terms of
how the partition functions of translation, vibration and rotation modes change from
those of the isolated reactants to those of the activated complex.
Additional insight into the physical basis of chemical reaction is obtained by applying
a thermodynamic formulation to activated complex theory. Equilibrium thermodynamics
shows that an equilibrium constant can be written in terms of the standard Gibbs free
energy (see Topics B6 and C1), which in this case is an activation Gibbs free energy,
‡
∆G , for formation of the activated complex, i.e.