Page 187 - Instant notes
P. 187
Energetics and mechanisms 173
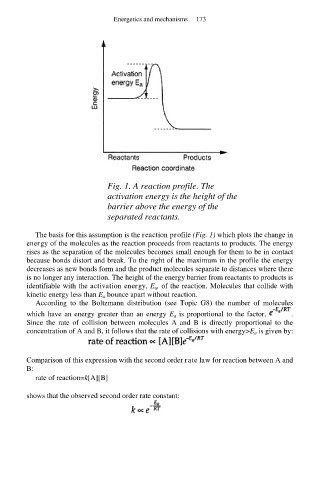
Fig. 1. A reaction profile. The
activation energy is the height of the
barrier above the energy of the
separated reactants.
The basis for this assumption is the reaction profile (Fig. 1) which plots the change in
energy of the molecules as the reaction proceeds from reactants to products. The energy
rises as the separation of the molecules becomes small enough for them to be in contact
because bonds distort and break. To the right of the maximum in the profile the energy
decreases as new bonds form and the product molecules separate to distances where there
is no longer any interaction. The height of the energy barrier from reactants to products is
identifiable with the activation energy, E a, of the reaction. Molecules that collide with
kinetic energy less than E a bounce apart without reaction.
According to the Boltzmann distribution (see Topic G8) the number of molecules
which have an energy greater than an energy E a is proportional to the factor, .
Since the rate of collision between molecules A and B is directly proportional to the
concentration of A and B, it follows that the rate of collisions with energy>E a is given by:
Comparison of this expression with the second order rate law for reaction between A and
B:
rate of reaction=k[A][B]
shows that the observed second order rate constant: