Page 267 - Instrumentation Reference Book 3E
P. 267
Measurement techniques: direct effects 251
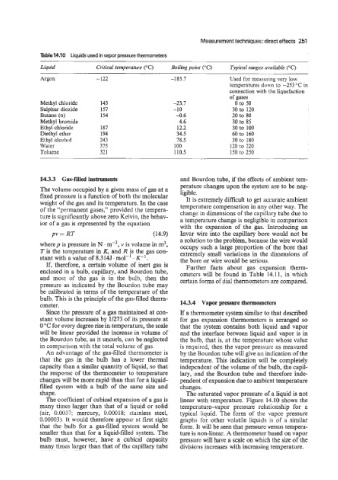
Table 14.10 Liquids used in vapor pressure thermometers
Liquid Critical temperature (“C) Boiling point (“C) Tvpical ranges mailable (“C)
Argon - -122 -185.7 Used for measuring very low
temperatures down to -253 ‘C in
connection with the liquefaction
of gases
Methyl chloride 143 -23.7 0 to 50
Sulphur dioxide 157 -10 30 to 120
Butane (n) 154 -0.6 20 to 80
Methyl brornide 4.6 30 to 85
Ethyl chloride 187 12.2 30 to 100
Diethyl ether 194 34.5 60 to 160
Ethyl alcohol 243 78.5 30 to 180
Water 375 100 120 to 220
Toluene 32 1 110.5 150 to 250
14.3.3 Gas-fded instruments and Bourdon tube, if the effects of ambient tem-
The volume occupied by a given mass of gas at a perature changes upon the system are to be neg-
ligible.
fixed pressure is a function of both the molecular
weight of the gas and its temperature. In the case It is extremely difficult to get accurate ambient
of the “permanent gases,” provided the tempera- temperature compensation in any other way. The
ture is significantly above zero Kelvin, the behav- change in dimensions of the capillary tube due to
ior of a gas is represented by the equation a temperature change is negligible in comparison
with the expansion of the gas. Introducing an
p~ = RT (14.9) Invar wire into the capillary bore would not be
a solution to the problem, because the wire would
where p is pressure in N . m-’, v is volume in m3, occupy such a large proportion of the bore that
Tis the temperature in K, and R is the gas con- extremely small variations in the dimensions of
stant with a value of 8.314J. mol-’ . K-’. the bore or wire would be serious.
If. therefore, a certain volume of inert gas is
Further facts about gas expansion therm-
enclosed in a bulb, capillary, and Bourdon tube, ometers will be found in Table 14.1 1, in which
and most of the gas is in the bulb, then the certain forms of dial thermometers are compared.
pressure as indicated by the Bourdon tube may
be calibrated in terms of the temperature of the
bulb. This is the principle of the gas-filled therm-
ometer. 14.3.4 Vapor pressure thermometers
Since the pressure of a gas maintained at con- If a thermometer system similar to that described
stant volume increases by 1/273 of its pressure at for gas expansion thermometers is arranged so
0 “C for every degree rise in temperature, the scale that the system contains both liquid and vapor
will be linear provided the increase in volume of and the interface between liquid and vapor is in
the Bourdon tube, as it uncurls, can be neglected the bulb, that is, at the temperature whose value
in comparison with the total volume of gas. is required, then the vapor pressure as measured
An advantage of the gas-filled thermometer is by the Bourdon tube will give an indication of the
that the gas in the bulb has a lower thermal temperature. This indication will be completely
capacity than a similar quantity of liquid, so that independent of the volume of the bulb, the capil-
the response of the thermometer to temperature lary, and the Bourdon tube and therefore inde-
changes will be more rapid,than that for a liquid- pendent of expansion due to ambient temperature
filled system with a bulb of the same size and changes.
shape. The saturated vapor pressure of a liquid is not
The coefficient of cubical expansion of a gas is linear with temperature. Figure 14.10 shows the
many times larger than that of a liquid or solid temperature-vapor pressure relationship for a
(air, 0.0037; mercury, 0.00018: stainless steel, typical liquid. The form of the vapor pressure
0.00003). It would therefore appear at first sight graphs for other volatile liquids is of a similar
that the bulb for a gas-filled system would be form. It will be seen that pressure versus tempera-
smaller than that for a liquid-filled system. The ture is non-linear. A thermometer based on vapor
bulb must, however, have a cubical capacity pressure will have a scale on which the size of the
many times larger than that of the capillary tube divisions increases with increasing temperature.