Page 335 - Instrumentation Reference Book 3E
P. 335
16 Chemical analysis:
spectroscopy
A. C. SMITH, edited by I.VERHAPPEN
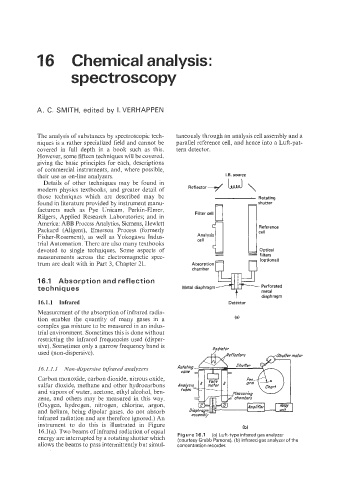
The analysis of substances by spectroscopic tech- taneously through an analysis cell assembly and a
niques is a rather specialized field and cannot be parallel reference cell, and hence into a Luft-pat-
covered in full depth in a book such as this. tern detector.
However, some fifteen techniques will be covered,
giving the basic principles for each, descriptions
of commercial instruments, and, where possible,
their use as on-line analyzers. I.R. source
Details of other techniques may be found in kceel \
modern physics textbooks, and greater detail of Reflector+
those techniques which are described may be Rotating
found in literature provided by instrument manu- shutter
facturers such as Pye Unicam, Perkin-Elmer,
Rilgers, Applied Research Laboratories; and in Filter cell
America: ABB Process Analytics, Siemens, Hewlett Ell
Packard (Aligent), Emerson Process (formerly Reference
cell
Fisher-Rosement), as well as Yokogawa Indus- Analysis
trial Automation. There are also many textbooks cell
devoted to single techniques. Some aspects of - -
Optical
measurements across the electromagnetic spec- filters
(opt iona I I
trum are dealt with in Part 3, Chapter 21. Absorption
chamber
16.1 Absorption and reflection
techniques Metal diaphragm Perforated
metal
diaphragm
16.1.1 Infrared Detector
Measurement of the absorption of infrared radia-
tion enables the quantity of many gases in a
complex gas mixture to be measured in an indus-
trial environment. Sometimes this is done without
restricting the infrared frequencies used (disper-
sive). Sometimes only a narrow frequency band is Radiofor
used (non-dispersive).
16. I. 1. I Non-dispersive inpared ana1,vzers
Carbon monoxide, carbon dioxide, nitrous oxide,
sulfur dioxide, methane and other hydrocarbons
and vapors of water, acetone, ethyl alcohol, ben-
zene, and others may be measured in this way.
(Oxygen, hydrogen, nitrogen, chlorine, argon,
and helium, being dipolar gases, do not absorb
infrared radiation and are therefore ignored.) An
instrument to do this is illustrated in Figure (b)
16.1(0 Two bemls of infrared radiation of equal
are interrupted by a rotating shutter which Figure 16.7 (a) Luft.typei"frared gasanalyzer
(courtesy Grubb Parsons), (b) infrared gas analyzer of the
allows the beams to pass intermittently but simul- concentration recorder.