Page 401 - Instrumentation Reference Book 3E
P. 401
384 Chemical analysis: gas analysis
contained in an environment whose temperature rate of the carrier gas, and these conditions must
can be held at a constant known value or heated be optimized for a particular analysis.
and cooled at controlled rates. The column may The composition of the gas passing through the
be uniformly packed with the granular stationary detector alternates between pure carrier gas and
phase (packed column chromatography), and this mixtures of the carrier gas with each of the com-
is normally used in process instruments. How- ponents of the sample. The output record of the
ever, it has been found that columns of the high- detector, known as the chromatogram, is a series
est separating performance are obtained if the of deflections or peaks, spaced in time and each
column is in the form of a capillary tube, with related to a component of the mixture analyzed.
the solid or liquid stationary phase coated on its A typical chromatogram of a mixture contain-
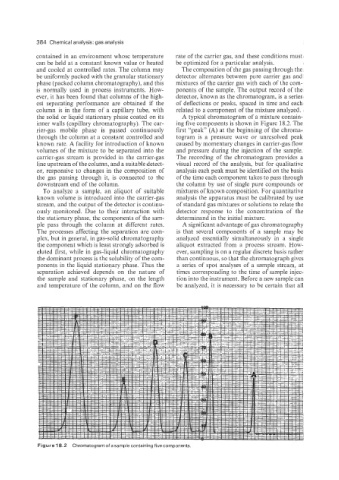
inner walls (capillary chromatography). The car- ing five components is shown in Figure 18.2. The
rier-gas mobile phase is passed continuously first “peak” (A) at the beginning of the chroma-
through the column at a constant controlled and togram is a pressure wave or unresolved peak
known rate. A facility for introduction of known caused by momentary changes in carrier-gas flow
volumes of the mixture to be separated into the and pressure during the injection of the sample.
carrier-gas stream is provided in the carrier-gas The recording of the chromatogram provides a
line upstream of the column, and a suitable detect- visual record of the analysis, but for qualitative
or, responsive to changes in the composition of analysis each peak must be identified on the basis
the gas passing through it, is connected to the of the time each component takes to pass through
downstream end of the column. the column by use of single pure compounds or
To analyze a sample, an aliquot of suitable mixtures of known composition. For quantitative
known volume is introduced into the carrier-gas analysis the apparatus must be calibrated by use
stream, and the output of the detector is continu- of standard gas mixtures or solutions to relate the
ously monitored. Due to their interaction with detector response to the concentration of the
the stationary phase, the components of the sam- determinand in the initial mixture.
ple pass through the column at different rates. A significant advantage of gas chromatography
The processes affecting the separation are com- is that several components of a sample may be
plex, but in general, in gas-solid chromatography analyzed essentially simultaneously in a single
the component which is least strongly adsorbed is aliquot extracted from a process stream. How-
eluted first, while in gas-liquid chromatography ever, sampling is on a regular discrete basis rather
the dominant process is the solubility of the com- than continuous, so that the chromatograph gives
ponents in the liquid stationary phase. Thus the a series of spot analyses of a sample stream, at
separation achieved depends on the nature of times corresponding to the time of sample injec-
the sample and stationary phase, on the length tion into the instrument. Before a new sample can
and temperature of the column, and on the flow be analyzed, it is necessary to be certain that all
Figure 18.2 Chromatogram of a sample containing five components.