Page 406 - Instrumentation Reference Book 3E
P. 406
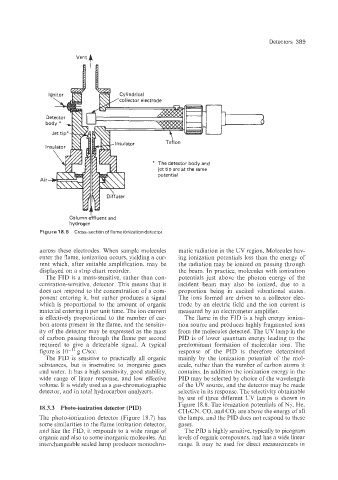
Detectors 389
Vent 4
Cy I ind rica I
'collector electrode
Teilon
I
* The detector body and
jet tip are at the same
potential
Ail
I
Column effluent and
hydrogen
Figure 18.6 Cross-section offlame ionization detector.
across these electrodes. When sample molecules matic radiation in the UV region. Molecules hav-
enter the flame, ionization occurs, yielding a cur- ing ionization potentials less than the energy of
rent which, after suitable amplification, may be the radiation may be ionized on passing through
displayed on a strip chart recorder. the beam. In practice, molecules with ionization
The FID is a mass-sensitive, rather than con- potentials just above the photon energy of the
centration-sensitive, detector. This means that it incident beam may also be ionized, due to a
does not respond to the concentration of a com- proportion being in excited vibrational states.
ponent entering it, but rather produces a signal The ions formed are driven to a collector elec-
which is proportional to the amount of organic trode by an electric field and the ion current is
material entering it per unit time. The ion current measured by an electrometer amplifier.
is effectively proportional to the number of car- The flame in the FID is a high energy ioniza-
bon atoms present in the flame, and the sensitiv- tion source and produces highly fragmented ions
ity of the detector may be expressed as the mass from the molecules detected. The UV lamp in the
of carbon passing through the flame per second PID is of lower quantum energy leading to the
required to give a detectable signal. A typical predominant formation of molecular ions. The
figure is 1W" g Clsec. response of the PID is therefore determined
The FID is sensitive to practically all organic mainly by the ionization potential of the mol-
substances, but is insensitive to inorganic gases ecule, rather than the number of carbon atoms it
and water. It has a high sensitivity. good stability. contains. In addition the ionization energy in the
wide range of hear response, and low effective PID may be selected by choice of the wavelength
volume. It is widely used as a gas-chromatographic of the UV source, and the detector may be made
detector, and in total hydrocarbon analyzers. selective in its response. The selectivity obtainable
by use of three different UV lamps is shown in
Figure 18.8. The ionization potentials of Nz. He,
18.3.3 Photo-ionization detector (PID)
CH3CN, CO, and COz are above the energy of all
The photo-ionization detector (Figure 18.7) has the lamps. and the PID does not respond to these
some similarities to the flame ionization detector, gases.
and like the FID, it responds to a wide range of The PID is highly sensitive, typically to picogram
organic and also to some inorganic molecules. An levels of organic compounds. and has a wide linear
interchangeable sealed lamp produces monochro- range. It may be used for direct measurements in