Page 410 - Instrumentation Reference Book 3E
P. 410
Detectors 393
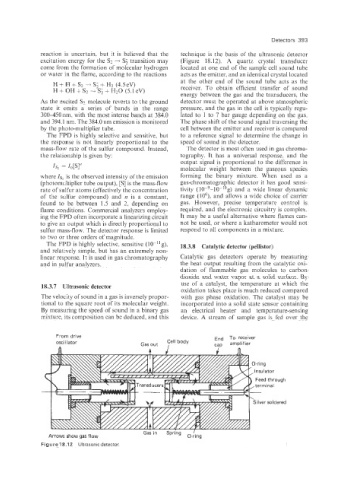
reaction is uncertain, but it is believed that the technique is the basis of the ultrasonic detector
excitation energy for the SZ + S; transition may (Figure 18.12). A quartz crystal transducer
come from -the formation of molecular hydrogen located at one end of the sample cell sound tube
or water in the flame, according to the reactions acts as the emitter, and an identical crystal located
H + H + S2 4 + H2 (4.5 eV) at the other end of the sound tube acts as the
S"
receiver. To obtain efficient transfer of sound
H + OH + S? +*S; + H20 (5.1 eV)
energy between the gas and the transducers, the
As the excited SZ. molecule reverts to the ground detector must be operated at above atmospheric
state it emits a series of bands in the range pressure, and the gas in the cell is typically regu-
300-450 nm, with the most intense bands at 384.0 lated to 1 to 7 bar gauge depending on the gas.
and 394.1 nm. The 384.0 nm emission is monitored The phase shift of the sound signal traversing the
by the photomultiplier tube. cell between the emitter and receiver is cornpared
The FPD is highly selective and sensitive, but to a reference signal to determine the change in
the response is not linearly proportional to the speed of sound in the detector.
mass-flow rate of the sulfur compound. Instead, The detector is most often used in gas chroma-
the relationship is given by: tography. It has a universal response, and the
output signal is proportional to the difference in
molecular weight between the gaseous species
where Is2 is the observed intensity of the emission forming the binary mixture. When used as a
(photoincltiplier tube output), [SI is the mass-flow gas-chromatographic detector it has good sensi-
rate of su!fur atoms (effectively the concentration tivity (10-9-10-'0g) and a wide linear dynamic
of the sulfur compound) and i? is a constant, range (lo6), and allows a wide choice of carrier
found to be between 1.5 and 2, depending on gas. However, precise temperature control is
flame conditions. Commercial analyzers employ- required, and the electronic circuitry is complex.
ing the FPD often incorporate a linearizing circuit It may be a useful alternative where flames can-
to give an output which is directly proportional to not be used, or where a katharorneter would not
sulfur mass-flow. The detector response is limited respond to ail components in a mixture.
to two or three orders of magnitude.
The FPD is highly selective, sensitive (lO-"g), 18.3.8 Catalytic detector (pellistor)
and relatively simple, but has an extremely non-
linear response. It is used in gas chromatography Catalytic gas detectors operate by measuring
and in sulfur analyzers. the heat output resulting from the catalytic oxi-
dation of flammable gas molecules to carbon
dioxide and water vapor at a solid surface. By
use of a catalyst, the temperature at which the
18.3.7 Ultrasonic detector
oxidation takes place is much reduced compared
The velocity of sound in a gas is inversely propor- with gas phase oxidation. The catalyst may be
tional to the square root of its molecular weight. incorporated into a solid state sensor containing
By measuring the speed of sound in a binary gas an electrical heater and temperature-sensing
mixture, its composition can be deduced, and this device. A stream of sample gas is fed over the
From drive End To receiver
oscillator Gasout Cell body ca(3 amplifier
,
Gasin Spring
Arrows show gas flow O-ring
Figure 18.12 Ultrasonic detector.