Page 411 - Instrumentation Reference Book 3E
P. 411
394 Chemical analysis: gas analysis
sensor, and flammable gases in the sample are Element 1
continuously oxidized, releasing heat and raising compensator
the temperature of the sensor. Temperature
variations in the sensor are monitored to give a
continuous record of the flammable-gas concen-
tration in the sample.
The most suitable metals for promoting the D.C.
oxidation of molecules containing C-H bonds,
such as methane and other organic species, are
those in Group 8 of the Periodic Table, particu-
larly platinum and palladium. The temperature Element 2/
sensor is usually a platinum resistance thermo-
meter, wound in a coil and also used as the elec-
trical heater for the sensor. The resistance is
measured by connecting the sensor as one arm
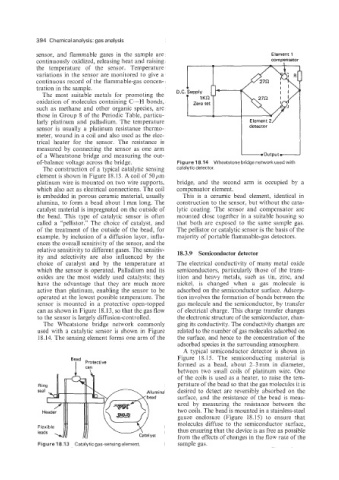
of a Wheatstone bridge and measuring the out-
of-balance voltage across the bridge. Figure 18.14 Wheatstone bridge networkused with
The construction of a typical catalytic sensing catalytic detector.
element is shown in Figure 18.13. A coil of 50pm
platinum wire is mounted on two wire supports, bridge, and the second arm is occupied by a
which also act as electrical connections. The coil compensator element.
is embedded in porous ceramic material, usually This is a ceramic bead element, identical in
alumina, to form a bead about lmm long. The construction to the sensor, but without the cata-
catalyst material is impregnated on the outside of lytic coating. The sensor and compensator are
the bead. This type of catalytic sensor is often mounted close together in a suitable housing so
called a “pellistor.” The choice of catalyst, and that both are exposed to the same sample gas.
of the treatment of the outside of the bead, for The pellistor or catalytic sensor is the basis of the
example, by inclusion of a diffusion layer, influ- majority of portable flammable-gas detectors.
ences the overall sensitivity of the sensor, and the
relative sensitivity to different gases. The sensitiv- 18.3.9 Semiconductor detector
ity and selectivity are also influenced by the
choice of catalyst and by the temperature at The electrical conductivity of many metal oxide
which the sensor is operated. Palladium and its semiconductors, particularly those of the trans-
oxides are the most widely used catalysts; they ition and heavy metals, such as tin, zinc, and
have the advantage that they are much more nickel, is changed when a gas molecule is
active than platinum, enabling the sensor to be adsorbed on the semiconductor surface. Adsorp-
operated at the lowest possible temperature. The tion involves the formation of bonds between the
sensor is mounted in a protective open-topped gas molecule and the semiconductor, by transfer
can as shown in Figure 18.13, so that the gas flow of electrical charge. This charge transfer changes
to the sensor is largely diffusion-controlled. the electronic structure of the semiconductor, chan-
The Wheatstone bridge network commonly ging its conductivity. The conductivity changes are
used with a catalytic sensor is shown in Figure related to the number of gas molecules adsorbed on
18.14. The sensing element forms one arm of the the surface, and hence to the concentration of the
adsorbed species in the surrounding atmosphere.
A typical semiconductor detector is shown in
Figure 18.15. The semiconducting material is
formed as a bead, about 2-3mm in diameter,
between two small coils of platinum wire. One
of the coils is used as a heater, to raise the tem-
perature of the bead so that the gas molecules it is
desired to detect are reversibly absorbed on the
surface, and the resistance of the bead is meas-
ured by measuring the resistance between the
two coils. The bead is mounted in a stainless-steel
gauze enclosure (Figure 18.15) to ensure that
molecules diffuse to the semiconductor surface,
thus ensuring that the device is as free as possible
from the effects of changes in the flow rate of the
Figure 18.13 Catalytic gas-sensing element. sample gas.