Page 239 - Introduction to Colloid and Surface Chemistry
P. 239
228 Colloid stability
150
100
>
E
10- 4 10-3 ID' 2 10- 1
Coagulation concentration/mol dnrr 3
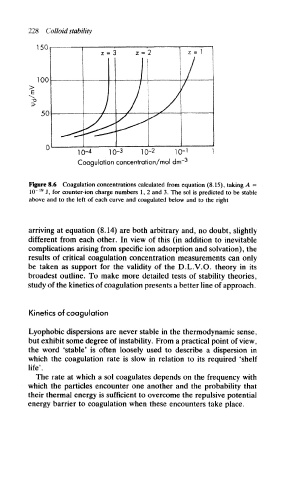
Figure 8.6 Coagulation concentrations calculated from equation (8.15), taking A -
19
10" J, for counter-ion charge numbers 1, 2 and 3. The sol is predicted to be stable
above and to the left of each curve and coagulated below and to the right
arriving at equation (8.14) are both arbitrary and, no doubt, slightly
different from each other. In view of this (in addition to inevitable
complications arising from specific ion adsorption and solvation), the
results of critical coagulation concentration measurements can only
be taken as support for the validity of the D.L.V.O. theory in its
broadest outline. To make more detailed tests of stability theories,
study of the kinetics of coagulation presents a better line of approach.
Kinetics of coagulation
Lyophobic dispersions are never stable in the thermodynamic sense,
but exhibit some degree of instability. From a practical point of view,
the word 'stable' is often loosely used to describe a dispersion in
which the coagulation rate is slow in relation to its required 'shelf
life'.
The rate at which a sol coagulates depends on the frequency with
which the particles encounter one another and the probability that
their thermal energy is sufficient to overcome the repulsive potential
energy barrier to coagulation when these encounters take place.