Page 242 - Introduction to Colloid and Surface Chemistry
P. 242
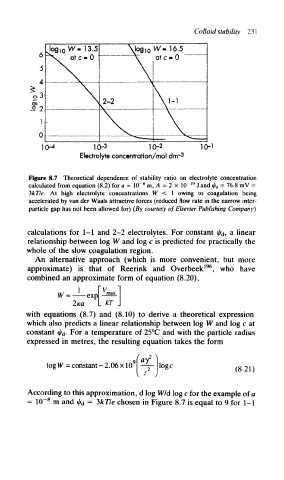
Colloid stability 231
10- 4 10-3 10-2 10-1
Electrolyte concentration/mol dm-3
Figure 8.7 Theoretical dependence of stability ratio on electrolyte concentration
8
calculated from equation (8.2) for a = 1(T m, A = 2 x 10 19 J and ty d = 76.8 mV =
3kTle. At high electrolyte concentrations W < 1 owing to coagulation being
accelerated by van der Waals attractive forces (reduced flow rate in the narrow inter-
particle gap has not been allowed for) (By courtesy of Elsevier Publishing Company)
calculations for 1-1 and 2-2 electrolytes. For constant fa, a linear
relationship between log W and log c is predicted for practically the
whole of the slow coagulation region.
An alternative approach (which is more convenient, but more
approximate) is that of Reerink and Overbeek 196 , who have
combined an approximate form of equation (8,20),
1
-exp
2/Cfl
with equations (8.7) and (8.10) to derive a theoretical expression
which also predicts a linear relationship between log W and log c at
constant fa. For a temperature of 25°C and with the particle radius
expressed in metres, the resulting equation takes the form
log W = constant -2. 06x10 logc
(8.21)
According to this approximation, d log W/d log c for the example of a
8
= 10~ m and fa = 3kT/e chosen in Figure 8.7 is equal to 9 for 1-1