Page 241 - Introduction to Colloid and Surface Chemistry
P. 241
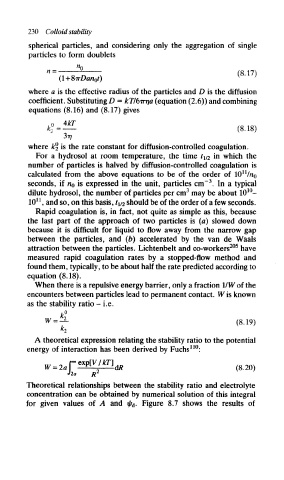
230 Colloid stability
spherical particles, and considering only the aggregation of single
particles to form doublets
where a is the effective radius of the particles and D is the diffusion
coefficient. Substituting D = kTI6rrrja (equation (2.6)) and combining
equations (8.16) and (8.17) gives
(8.18)
where fcj> is the rate constant for diffusion-controlled coagulation.
For a hydrosol at room temperature, the time f 1/2 in which the
number of particles is halved by diffusion-controlled coagulation is
H
calculated from the above equations to be of the order of 10 /n 0
3
seconds, if « 0 is expressed in the unit, particles cm" . In a typical
3
10
dilute hydrosol, the number of particles per cm may be about 10 -
11
10 , and so, on this basis, * 1/2 should be of the order of a few seconds.
Rapid coagulation is, in fact, not quite as simple as this, because
the last part of the approach of two particles is (a) slowed down
because it is difficult for liquid to flow away from the narrow gap
between the particles, and (b) accelerated by the van de Waals
205
attraction between the particles. Lichtenbelt and co-workers have
measured rapid coagulation rates by a stopped-flow method and
found them, typically, to be about half the rate predicted according to
equation (8.18).
When there is a repulsive energy barrier, only a fraction l/Wof the
encounters between particles lead to permanent contact. W is known
as the stability ratio - i.e.
k°
W = -^~ (8.19)
k 2
A theoretical expression relating the stability ratio to the potential
110
energy of interaction has been derived by Fuchs :
a (8.20)
Theoretical relationships between the stability ratio and electrolyte
concentration can be obtained by numerical solution of this integral
for given values of A and tf/ d. Figure 8.7 shows the results of