Page 247 - Introduction to Colloid and Surface Chemistry
P. 247
236 Colloid stability
electrolyte concentration is often sufficiently high to render electro-
static stabilisation ineffective.
Effect on electric double layer interactions
If the stabilising agent is ionised and carries a charge of the same sign
as that on the particles (e.g. anionic surfactant adsorbed on
negatively charged particles), then electric double layer repulsion will
be enhanced. The adsorbed stabilising agent (even if non-ionic) will
influence electrostatic interactions by causing a displacement of the
Stern plane away from the particle surface; this will increase the
range of electric double layer repulsion and, as such, enhance
stability.
Effect on van der Waals interactions
Adsorbed layers of stabilising agent may cause a significant lowering
of the effective Hamaker constant and, therefore, a weakening of the
interparticle van der Waals attraction. This effect has been considered
by Void 207 and by Vincent and co-workers 208 in terms of the
Hamaker microscopic treatment of dispersion forces.
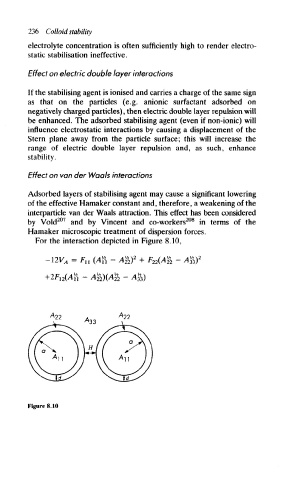
For the interaction depicted in Figure 8.10,
— 12V'A = Fit (Aii — A*)->\ + F-y?(Ay> — A-IT.)
Figure 8.10