Page 251 - Introduction to Colloid and Surface Chemistry
P. 251
240 Colloid stability
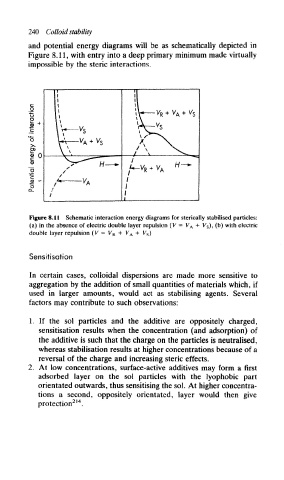
and potential energy diagrams will be as schematically depicted in
Figure 8.11, with entry into a deep primary minimum made virtually
impossible by the steric interactions.
Figure 8.11 Schematic interaction energy diagrams for sterically stabilised particles:
(a) in the absence of electric double layer repulsion (V = V A + V s), (b) with electric
double layer repulsion (V — V R + V A + V s)
Sensitisation
In certain cases, colloidal dispersions are made more sensitive to
aggregation by the addition of small quantities of materials which, if
used in larger amounts, would act as stabilising agents. Several
factors may contribute to such observations:
1. If the sol particles and the additive are oppositely charged,
sensitisation results when the concentration (and adsorption) of
the additive is such that the charge on the particles is neutralised,
whereas stabilisation results at higher concentrations because of a
reversal of the charge and increasing steric effects.
2, At low concentrations, surface-active additives may form a first
adsorbed layer on the sol particles with the lyophobic part
orientated outwards, thus sensitising the sol. At higher concentra-
tions a second, oppositely orientated, layer would then give
protection 214 .