Page 97 - Introduction to Mineral Exploration
P. 97
80 C.J. MOON & M.K.G. WHATELEY
TABLE 5.3 Colours of minerals in outcrop.
Mineral or metal Outcrop color Mineral/compound in outcrop
Iron sulfides Yellows, browns, chestnuts, reds Goethite, hematite, limonite, sulfates
Manganese Blacks Mn oxides, wad
Antimony White Antimony bloom
Arsenic Greenish, greens, yellowish Iron arsenate
Bismuth Light yellow Bismuth ochres
Cadmium Light yellow Cadmium sulfide
Cobalt Black, pink, sometimes violet Oxides, erythrite
Copper Greens, blues Carbonates, silicates, sulfates, oxides, native Cu
Lead White, yellow Cerussite, anglesite, pyromorphite
Mercury Red Cinnabar
Molybdenum Bright yellow Molydenum oxides, iron molybdate
Nickel Green Annabergite, garnierite
Silver Waxy green, yellow Chlorides, native Ag
Uranium Bright green, yellow Torbernite, autunite
Vanadium Green, yellow Vanadates
Zinc White Smithsonite
multielement analysis, either XRF or ICP–ES,
(a) (b) (c)
following a total attack to dissolve silica. The
interpretation needs to be treated with care. A
large amount of money was wasted in West-
ern Australia at the height of the nickel boom
in the early 1970s because ironstones were
evaluated for potential on the basis of their
nickel content. While most ironstones with
very high nickel contents do overlie nickel
sulfide deposits, a number have scavenged
(d) (e) (f) the relatively mobile nickel from circulating
ground waters or overlie silicate sources of
nickel and a number of deposits have a weak
nickel expression at surface. More careful
research demonstrated that it is better to con-
sider the ratio of nickel to more immobile ele-
ments, such as copper, or even better immobile
iridium that is present at the sub-ppm level in
the deposits. These elements can be combined
(g) (h) (i) (j)
into a discriminant index (Travis et al. 1976,
Moeskops 1977). Similar considerations also
apply to base metal deposits; barium and lead
have been shown to be useful immobile tracers
in these (summarized in Butt & Zeegers 1992).
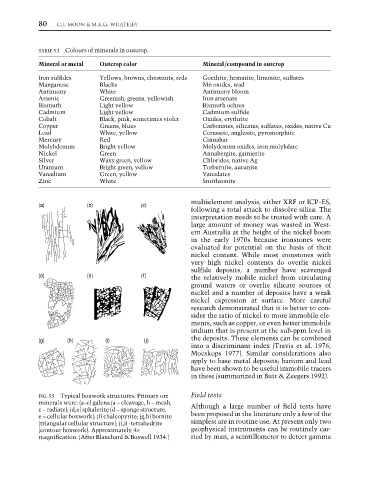
FIG. 5.5 Typical boxwork structures. Primary ore Field tests
minerals were: (a–c) galena (a – cleavage, b – mesh, Although a large number of field tests have
c – radiate); (d,e) sphalerite (d – sponge structure,
e – cellular boxwork); (f) chalcopyrite; (g,h) bornite been proposed in the literature only a few of the
(triangular cellular structure); (i,j) -tetrahedrite simplest are in routine use. At present only two
(contour boxwork). Approximately 4× geophysical instruments can be routinely car-
magnification. (After Blanchard & Boswell 1934.) ried by man, a scintillometer to detect gamma