Page 223 - Introduction to Petroleum Engineering
P. 223
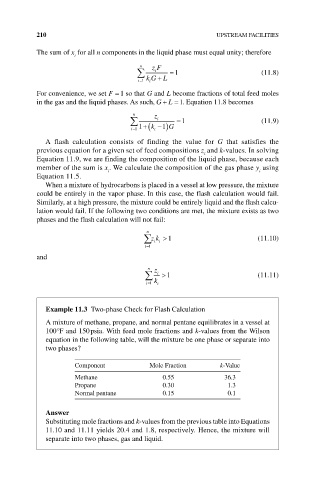
210 UPSTREAM FACILITIES
The sum of x for all n components in the liquid phase must equal unity; therefore
i
n zF
i 1 (11.8)
i 1 kG L
i
For convenience, we set F 1 so that G and L become fractions of total feed moles
in the gas and the liquid phases. As such, GL 1. Equation 11.8 becomes
n z
i 1 (11.9)
i 1 1 k i 1 G
A flash calculation consists of finding the value for G that satisfies the
previous equation for a given set of feed compositions z and k‐values. In solving
i
Equation 11.9, we are finding the composition of the liquid phase, because each
member of the sum is x . We calculate the composition of the gas phase y using
i
i
Equation 11.5.
When a mixture of hydrocarbons is placed in a vessel at low pressure, the mixture
could be entirely in the vapor phase. In this case, the flash calculation would fail.
Similarly, at a high pressure, the mixture could be entirely liquid and the flash calcu-
lation would fail. If the following two conditions are met, the mixture exists as two
phases and the flash calculation will not fail:
n
zk 1 (11.10)
ii
i 1
and
n z
i 1 (11.11)
i 1 k i
Example 11.3 Two‐phase Check for Flash Calculation
A mixture of methane, propane, and normal pentane equilibrates in a vessel at
100°F and 150 psia. With feed mole fractions and k‐values from the Wilson
equation in the following table, will the mixture be one phase or separate into
two phases?
Component Mole Fraction k‐Value
Methane 0.55 36.3
Propane 0.30 1.3
Normal pentane 0.15 0.1
Answer
Substituting mole fractions and k‐values from the previous table into Equations
11.10 and 11.11 yields 20.4 and 1.8, respectively. Hence, the mixture will
separate into two phases, gas and liquid.