Page 97 - Introduction to chemical reaction engineering and kinetics
P. 97
4.4 Dependence of Rate on Temperature 79
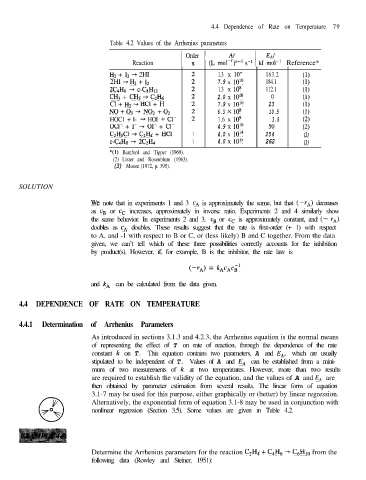
Table 4.2 Values of the Arrhenius parameters
I I
Order
Reaction n (L moljf:“l s-r 1kJ 2Ll-r 1 Reference*
H2 + I2 * 2HI 1.3 x 10” 163.2 (1)
2HI + H2 + I2 7.9 x 10’0 184.1 (1)
2C4H6 + c-&HI2 1.3 x 108 112.1 (1)
CH3 + CH3 + C2H6 2.0 x 10’0 0 (1)
Cl + H2 ---) HCl + H 7.9 x 10’0 23 (1)
NO+03 + NO2 +02 6.3 x 10s 10.5 (1)
HOC1 + I- + HOI + Cl- 1.6 x log 3.8 (2)
OCl- + I- + 01- + cl- 4.9 x 10’0 50 (2)
C2HsCl -+ C21-L, + HCl 1 4.0 x 10’4 254 (3)
c-C4Hs * 2C& 1 4.0 x 10’5 262 (3)
*(l) Bamford and Tipper (1969).
(2) Lister and Rosenblum (1963).
(3) Moore (1972, p. 395).
SOLUTION
We note that in experiments 1 and 3 CA is approximately the same, but that (-rA) decreases
as cn or cc increases, approximately in inverse ratio. Experiments 2 and 4 similarly show
the same behavior. In experiments 2 and 3, cu or co is approximately constant, and (- rA)
doubles as CA doubles. These results suggest that the rate is first-order (+ 1) with respect
to A, and -1 with respect to B or C, or (less likely) B and C together. From the data
given, we can’t tell which of these three possibilities correctly accounts for the inhibition
by product(s). However, if, for example, B is the inhibitor, the rate law is
(-YA) = kAcAc<’
and kA can be calculated from the data given.
4.4 DEPENDENCE OF RATE ON TEMPERATURE
4.4.1 Determination of Arrhenius Parameters
As introduced in sections 3.1.3 and 4.2.3, the Arrhenius equation is the normal means
of representing the effect of T on rate of reaction, through the dependence of the rate
constant k on T. This equation contains two parameters, A and EA, which are usually
stipulated to be independent of T. Values of A and EA can be established from a mini-
mum of two measurements of k at two temperatures. However, more than two results
are required to establish the validity of the equation, and the values of A and EA are
then obtained by parameter estimation from several results. The linear form of equation
3.1-7 may be used for this purpose, either graphically or (better) by linear regression.
Alternatively, the exponential form of equation 3.1-8 may be used in conjunction with
nonlinear regression (Section 3.5). Some values are given in Table 4.2.
Determine the Arrhenius parameters for the reaction C,H4 + C4H, + C6H,, from the
following data (Rowley and Steiner, 1951):