Page 96 - Introduction to chemical reaction engineering and kinetics
P. 96
78 Chapter 4: Development of the Rate Law for a Simple System
Developm
1 1
0.9
0.9
0.8
0.8
0.7
0.7
0.6
0.6
9 9
-L! 0.5
z
0.4
0.3
0.2
0 . 1
0
0 1 2 3 4 5 6 7 8 9 1 0
Mh
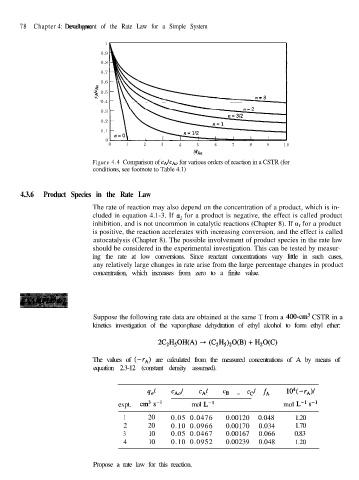
Figure 4.4 Comparison of CA/C..Q, for various orders of reaction in a CSTR (for
conditions, see footnote to Table 4.1)
4.3.6 Product Species in the Rate Law
The rate of reaction may also depend on the concentration of a product, which is in-
cluded in equation 4.1-3. If (Ye for a product is negative, the effect is called product
inhibition, and is not uncommon in catalytic reactions (Chapter 8). If oi for a product
is positive, the reaction accelerates with increasing conversion, and the effect is called
autocatalysis (Chapter 8). The possible involvement of product species in the rate law
should be considered in the experimental investigation. This can be tested by measur-
ing the rate at low conversions. Since reactant concentrations vary little in such cases,
any relatively large changes in rate arise from the large percentage changes in product
concentration, which increases from zero to a finite value.
Suppose the following rate data are obtained at the same T from a 400-cm3 CSTR in a
kinetics investigation of the vapor-phase dehydration of ethyl alcohol to form ethyl ether:
The values of (-T*) are calculated from the measured concentrations of A by means of
equation 2.3-12 (constant density assumed).
d cA,i cAJ % = cCJ fA lo4( --IA)/
expt. cm3 s-l mol L-l mol L-l s-l
1 20 0.05 0.0476 0.00120 0.048 1.20
2 20 0.10 0.0966 0.00170 0.034 1.70
3 10 0.05 0.0467 0.00167 0.066 0.83
4 10 0.10 0.0952 0.00239 0.048 1.20
Propose a rate law for this reaction.