Page 99 - Introduction to chemical reaction engineering and kinetics
P. 99
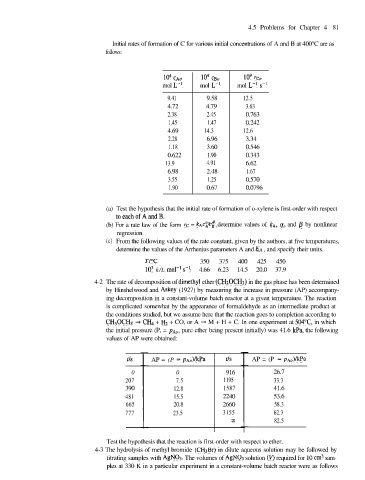
4.5 Problems for Chapter 4 81
Initial rates of formation of C for various initial concentrations of A and B at 400°C are as
follows:
I@ CAo 104 CBO 109 rcO
mol L-l mol L-l mol L-l s-l
9.41 9.58 12.5
4.72 4.79 3.63
2.38 2.45 0.763
1.45 1.47 0.242
4.69 14.3 12.6
2.28 6.96 3.34
1.18 3.60 0.546
0.622 1.90 0.343
13.9 4.91 6.62
6.98 2.48 1.67
3.55 1.25 0.570
1.90 0.67 0.0796
(a) Test the hypothesis that the initial rate of formation of o-xylene is first-order with respect
toeachofAandB.
a
(b) For a rate law of the form rc = kAcAcB , s determine values of kA, (Y, and /3 by nonlinear
regression.
(c) From the following values of the rate constant, given by the authors, at five temperatures,
determine the values of the Arrhenius parameters A and EA , and specify their units.
T/“C 350 375 400 425 450
lo3 k/L mol-i s-l 4.66 6.23 14.5 20.0 37.9
4-2 The rate of decomposition of dimethyl ether (CHsOCHs) in the gas phase has been determined
by Hinshelwood and Askey (1927) by measuring the increase in pressure (AP) accompany-
ing decomposition in a constant-volume batch reactor at a given temperature. The reaction
is complicated somewhat by the appearance of formaldehyde as an intermediate product at
the conditions studied, but we assume here that the reaction goes to completion according to
CH30CH3 -+ CHq + Hz + CO, or A + M + H + C. In one experiment at 504°C in which
the initial pressure (P, = PAo, pure ether being present initially) was 41.6 kPa, the following
values of AP were obtained:
AP = (P - pAo)kPa AP = (P - PAo)lkPa
0 0 916 26.7
207 7.5 1195 33.3
390 12.8 1587 41.6
481 15.5 2240 53.6
665 20.8 2660 58.3
777 23.5 3155 62.3
to 82.5
Test the hypothesis that the reaction is first-order with respect to ether.
4-3 The hydrolysis of methyl bromide (CHsBr) in dilute aqueous solution may be followed by
titrating samples with AgNOs. The volumes of AgNOs solution (V) required for 10 cm3 sam-
ples at 330 K in a particular experiment in a constant-volume batch reactor were as follows