Page 98 - Introduction to chemical reaction engineering and kinetics
P. 98
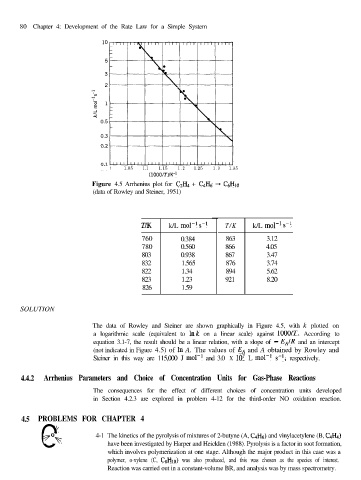
80 Chapter 4: Development of the Rate Law for a Simple System
1 1.05 1.1 1.15 1 . 2 1.25 1 . 3 1.35
(lOOO/T)/K-’
Figure 4.5 Arrhenius plot for C2H4 + CdHh -+ CeHto
(data of Rowley and Steiner, 1951)
T/K k/L mole1 s-l T/K k/L mole1 s-l
760 0.384 863 3.12
780 0.560 866 4.05
803 0.938 867 3.47
832 1.565 876 3.74
822 1.34 894 5.62
823 1.23 921 8.20
826 1.59
SOLUTION
The data of Rowley and Steiner are shown graphically in Figure 4.5, with k plotted on
a logarithmic scale (equivalent to Ink on a linear scale) against lOOO/T. According to
equation 3.1-7, the result should be a linear relation, with a slope of - E,IR and an intercept
(not indicated in Figure 4.5) of In A. The values of EA and A obtained by Rowley and
Steiner in this way are 115,000 J mol-’ and 3.0 X lo7 L mol-’ s-l, respectively.
4.4.2 Arrhenius Parameters and Choice of Concentration Units for Gas-Phase Reactions
The consequences for the effect of different choices of concentration units developed
in Section 4.2.3 are explored in problem 4-12 for the third-order NO oxidation reaction.
4.5 PROBLEMS FOR CHAPTER 4
0 4-1 The kinetics of the pyrolysis of mixtures of 2-butyne (A, C4H6) and vinylacetylene (B, Cab)
v
“O-v
have been investigated by Harper and Heicklen (1988). Pyrolysis is a factor in soot formation,
which involves polymerization at one stage. Although the major product in this case was a
polymer, o-xylene (C, CsHro) was also produced, and this was chosen as the species of interest.
Reaction was carried out in a constant-volume BR, and analysis was by mass spectrometry.