Page 298 - Lindens Handbook of Batteries
P. 298
13.4 PrImArY BATTErIES
1.7
Manufacturer A
1.6
1.5
Manufacturer B
Cell voltage, V 1.3 Manufacturer C
1.4
1.2
1.1
1.0
0.9
0 100 200 300 400 500 600 700
Hours on 33 kohms discharge
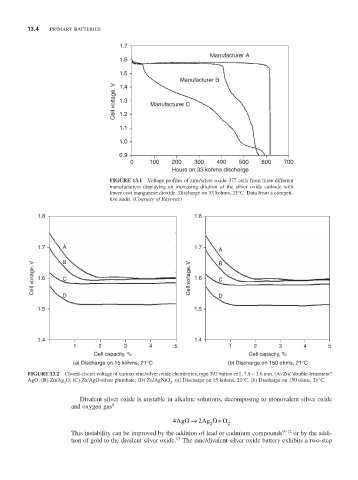
FiGURE 13.1 Voltage profiles of zinc/silver oxide 377 cells from three different
manufacturers displaying an increasing dilution of the silver oxide cathode with
lower cost manganese dioxide. Discharge on 33 kohms, 21°C. Data from a competi-
tive audit. (Courtesy of Rayovac)
1.8 1.8
1.7 A 1.7
A
B
B
Cell voltage, V 1.6 C Cell voltage, V 1.6 C
1.5 D 1.5 D
1.4 1.4
1 2 3 4 5 1 2 3 4 5
Cell capacity, % Cell capacity, %
(a) Discharge on 15 kohms, 21°C (b) Discharge on 150 ohms, 21°C
FiGURE 13.2 Closed-circuit voltage of various zinc/silver oxide chemistries, type 392 button cell, 7.8 × 3.6 mm. (A) Zn/“double-treatment”
AgO; (B) Zn/Ag O; (C) Zn/AgO-silver plumbate; (D) Zn/AgniO . (a) Discharge on 15 kohms, 21°C. (b) Discharge on 150 ohms, 21°C.
2 2
Divalent silver oxide is unstable in alkaline solutions, decomposing to monovalent silver oxide
and oxygen gas 8
+
4AgO → 2AgO O
2
2
This instability can be improved by the addition of lead or cadmium compounds 9–12 or by the addi-
13
tion of gold to the divalent silver oxide. The zinc/divalent silver oxide battery exhibits a two-step