Page 301 - Lindens Handbook of Batteries
P. 301
BUTTOn CELL BATTErIES: SILVEr OxIDE–ZInC AnD ZInC-AIr SYSTEmS 13.7
A second approach to eliminate the two-step discharge was through the use of silver plumbate as
17
a cathode additive material. Silver plumbate cathode material was prepared by reacting an excess
of divalent silver oxide as a coarse powder with lead sulfide (PbS) in a hot alkaline solution. The
product of the reaction is a mixture of remaining divalent silver oxide (AgO), monovalent silver
oxide (Ag O), and silver plumbate (Ag Pb O ). The sulfur is oxidized to the sulfate and is washed
2
5
6
2
from the reaction product
2PbS+ 19AgO+ 4naOH → Ag Pb O + 5 2 6 7AgO+ 2 2na SO + 2 4 2HO
2
The AgO particles after the reaction retain a core of AgO but have an outer coating of monovalent
silver oxide and silver plumbate. The silver plumbate compound is conductive, stable, and cathode
active. The Ag O serves to mask the AgO, while the conductive Ag Pb O improves the cell imped-
2
6
5
2
ance. Unlike silver metal, the conductive silver plumbate is not oxidized by the AgO and the cathode
impedance remains low throughout the cell life.
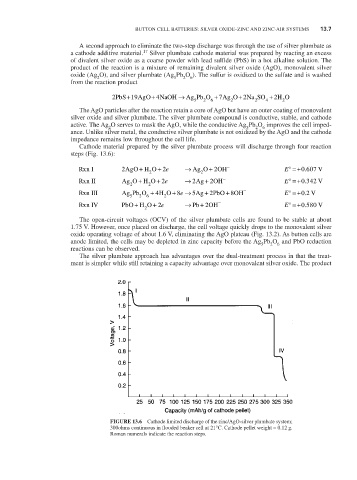
Cathode material prepared by the silver plumbate process will discharge through four reaction
steps (Fig. 13.6):
+
+
+
rxnI 2 AgOH O 2e → AgO 2 OH - E° =+ 0 607 V
.
2 2
rxnII AgO H O + + O 2e → 2 Ag + OH - E° = 2 +0 342. V
2 2
rxn III Ag Pb O + 5 2 6 H O + 4 2 8e → 5Ag + 5 2PbO + 8OH - E° =+ 0 2 V.
+
.
rxnIV PbO HO + 2e → Pb + 2OH - E° ==+0 580 V
2
The open-circuit voltages (OCV) of the silver plumbate cells are found to be stable at about
1.75 V. However, once placed on discharge, the cell voltage quickly drops to the monovalent silver
oxide operating voltage of about 1.6 V, eliminating the AgO plateau (Fig. 13.2). As button cells are
anode limited, the cells may be depleted in zinc capacity before the Ag Pb O and PbO reduction
5
6
2
reactions can be observed.
The silver plumbate approach has advantages over the dual-treatment process in that the treat-
ment is simpler while still retaining a capacity advantage over monovalent silver oxide. The product
FiGURE 13.6 Cathode limited discharge of the zinc/AgO-silver plumbate system;
300ohms continuous in flooded beaker cell at 21°C. Cathode pellet weight = 0.12 g.
roman numerals indicate the reaction steps.