Page 352 - Lindens Handbook of Batteries
P. 352
LiTHiUM PriMAry BATTerieS 14.17
As lithium reacts readily with water, a nonaqueous elec- 6
trolyte consisting of sulfur dioxide and an organic solvent,
typically acetonitrile, with dissolved lithium bromide is
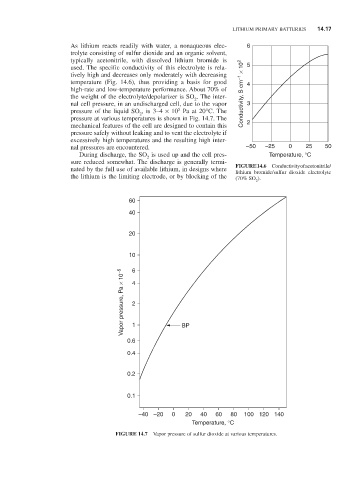
used. The specific conductivity of this electrolyte is rela- 5
tively high and decreases only moderately with decreasing
temperature (Fig. 14.6), thus providing a basis for good 4
high-rate and low-temperature performance. About 70% of
the weight of the electrolyte/depolarizer is SO . The inter- Conductivity, S cm –1 × 10 2
2
nal cell pressure, in an undischarged cell, due to the vapor 3
5
pressure of the liquid SO , is 3–4 × 10 Pa at 20°C. The
2
pressure at various temperatures is shown in Fig. 14.7. The
mechanical features of the cell are designed to contain this 2
pressure safely without leaking and to vent the electrolyte if
excessively high temperatures and the resulting high inter-
nal pressures are encountered. –50 –25 0 25 50
During discharge, the SO is used up and the cell pres- Temperature, °C
2
sure reduced somewhat. The discharge is generally termi-
nated by the full use of available lithium, in designs where FIGURE 14.6 Conductivity of acetonitrile/
lithium bromide/sulfur dioxide electrolyte
the lithium is the limiting electrode, or by blocking of the (70% SO ).
2
60
40
20
10 6
Vapor pressure, Pa × 10 –5 4 2
0.6 1 BP
0.4
0.2
0.1
–40 –20 0 20 40 60 80 100 120 140
Temperature, °C
FIGURE 14.7 Vapor pressure of sulfur dioxide at various temperatures.