Page 399 - Lindens Handbook of Batteries
P. 399
14.64 PriMAry BATTerieS
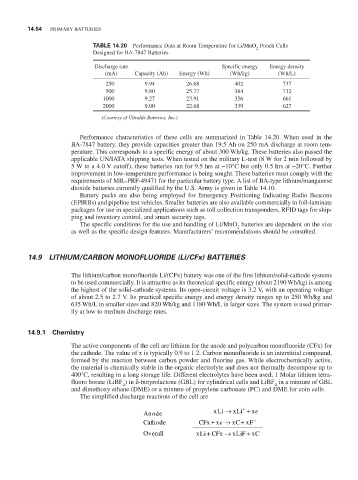
TABLE 14.20 Performance Data at room Temperature for Li/MnO Pouch Cells
2
Designed for BA-7847 Batteries
Discharge rate Specific energy energy density
(mA) Capacity (Ah) energy (Wh) (Wh/kg) (Wh/L)
250 9.94 26.68 402 737
500 9.80 25.77 384 712
1000 9.27 23.91 356 661
2000 9.00 22.68 339 627
(Courtesy of Ultralife Batteries, Inc.)
Performance characteristics of these cells are summarized in Table 14.20. When used in the
BA-7847 battery, they provide capacities greater than 19.5 Ah on 250 mA discharge at room tem-
perature. This corresponds to a specific energy of about 300 Wh/kg. These batteries also passed the
applicable UN/iATA shipping tests. When tested on the military L-test (8 W for 2 min followed by
5 W to a 4.0 V cutoff), these batteries ran for 9.5 hrs at -10°C but only 0.5 hrs at -20°C. Further
improvement in low-temperature performance is being sought. These batteries must comply with the
requirements of MiL-PrF-49471 for the particular battery type. A list of BA-type lithium/manganese
dioxide batteries currently qualified by the U.S. Army is given in Table 14.10.
Battery packs are also being employed for emergency Positioning indicating radio Beacons
(ePirBs) and pipeline test vehicles. Smaller batteries are also available commercially in foil-laminate
packages for use in specialized applications such as toll collection transponders, rFiD tags for ship-
ping and inventory control, and smart security tags.
The specific conditions for the use and handling of Li/MnO batteries are dependent on the size
2
as well as the specific design features. Manufacturers’ recommendations should be consulted.
14.9 LITHIUM/CARBON MONOFLUORIDE (Li/CFx) BATTERIES
The lithium/carbon monofluoride Li/(CFx) battery was one of the first lithium/solid-cathode systems
to be used commercially. it is attractive as its theoretical specific energy (about 2190 Wh/kg) is among
the highest of the solid-cathode systems. its open-circuit voltage is 3.2 V, with an operating voltage
of about 2.5 to 2.7 V. its practical specific energy and energy density ranges up to 250 Wh/kg and
635 Wh/L in smaller sizes and 820 Wh/kg and 1180 Wh/L in larger sizes. The system is used primar-
ily at low to medium discharge rates.
14.9.1 Chemistry
The active components of the cell are lithium for the anode and polycarbon monofluoride (CFx) for
the cathode. The value of x is typically 0.9 to 1.2. Carbon monofluoride is an interstitial compound,
formed by the reaction between carbon powder and fluorine gas. While electrochemically active,
the material is chemically stable in the organic electrolyte and does not thermally decompose up to
400°C, resulting in a long storage life. Different electrolytes have been used; 1 Molar lithium tetra-
fluoro borate (LiBF ) in δ-butyrolactone (GBL) for cylindrical cells and LiBF in a mixture of GBL
4
4
and dimethoxy ethane (DMe) or a mixture of propylene carbonate (PC) and DMe for coin cells.
The simplified discharge reactions of the cell are
Anode xLi → xLi + + xe
+
+
Cathode CFxx → e xC xF -
Overall xLi + CCFx → xLiFxC
+