Page 410 - Lindens Handbook of Batteries
P. 410
LiTHiUM PriMAry BATTerieS 14.75
3
2.5
2
Cell potential, V 1.5
1
0.5
C/40 rate, no predischarge
C/40 rate, predischarge
0
0 100 200 300 400 500 600 700 800
Capacity, mAh/g
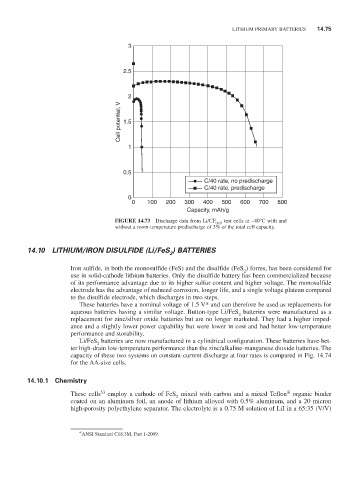
FIGURE 14.73 Discharge data from Li/CF 0.65 test cells at -40°C with and
without a room-temperature predischarge of 3% of the total cell capacity.
14.10 LITHIUM/IRON DISULFIDE (Li/FeS ) BATTERIES
2
iron sulfide, in both the monosulfide (FeS) and the disulfide (FeS ) forms, has been considered for
2
use in solid-cathode lithium batteries. Only the disulfide battery has been commercialized because
of its performance advantage due to its higher sulfur content and higher voltage. The monosulfide
electrode has the advantage of reduced corrosion, longer life, and a single voltage plateau compared
to the disulfide electrode, which discharges in two steps.
These batteries have a nominal voltage of 1.5 V* and can therefore be used as replacements for
aqueous batteries having a similar voltage. Button-type Li/FeS batteries were manufactured as a
2
replacement for zinc/silver oxide batteries but are no longer marketed. They had a higher imped-
ance and a slightly lower power capability but were lower in cost and had better low-temperature
performance and storability.
Li/FeS batteries are now manufactured in a cylindrical configuration. These batteries have bet-
2
ter high-drain low-temperature performance than the zinc/alkaline-manganese dioxide batteries. The
capacity of these two systems on constant-current discharge at four rates is compared in Fig. 14.74
for the AA-size cells.
14.10.1 Chemistry
53
®
These cells employ a cathode of FeS mixed with carbon and a mixed Teflon organic binder
2
coated on an aluminum foil, an anode of lithium alloyed with 0.5% aluminum, and a 20 micron
high-porosity polyethylene separator. The electrolyte is a 0.75 M solution of Lii in a 65:35 (V/V)
* ANSi Standard C18.3M, Part 1-2009.