Page 411 - Lindens Handbook of Batteries
P. 411
14.76 PriMAry BATTerieS
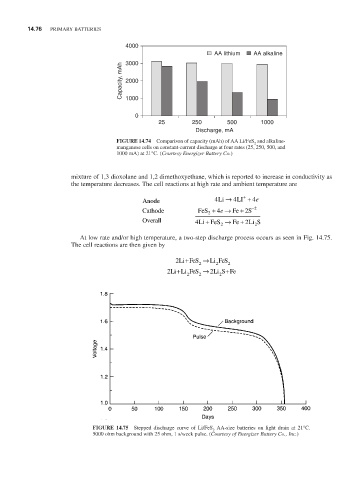
4000
AA lithium AA alkaline
3000
Capacity, mAh 2000
1000
0
25 250 500 1000
Discharge, mA
FIGURE 14.74 Comparison of capacity (mAh) of AA Li/FeS and alkaline-
2
manganese cells on constant-current discharge at four rates (25, 250, 500, and
1000 mA) at 21°C. (Courtesy Energizer Battery Co.)
mixture of 1,3 dioxolane and 1,2 dimethoxyethane, which is reported to increase in conductivity as
the temperature decreases. The cell reactions at high rate and ambient temperature are
Anode 4 Li → 4 Li + + 4e
+
Cathode FeS + 2 4 → e Fe 2 S - -2
Overall + 4Li FeS 2 → Fe + 2LiS
2
At low rate and/or high temperature, a two-step discharge process occurs as seen in Fig. 14.75.
The cell reactions are then given by
+
2Li FeS → LiFeS
2 2 2
+
+
2Li Li FeS → 2 2 2LiS Fe
2
FIGURE 14.75 Stepped discharge curve of Li/FeS AA-size batteries on light drain at 21°C.
2
5000 ohm background with 25 ohm, 1 s/week pulse. (Courtesy of Energizer Battery Co., Inc.)